Key takeaways:
~The NQO1 gene codes for a phase II detoxification enzyme that breaks down quinones, benzene, and some specific chemotherapy drugs, acting as a reducing agent to make certain substances easier for the body to eliminate.
~ It is also important in metabolizing and removing estrogen quinone metabolites, which are linked to breast cancer risk.
~ Genetic variants in the NQO1 gene can change its function, increasing or decreasing your risk of cancer or other negative effects from toxins.
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Join today.
NQO1 gene:
Listen to this article:
Your genes code for the enzymes that break down the toxic substances we all encounter each day. The ability to clear out potential carcinogens is important when it comes to preventing cancer.
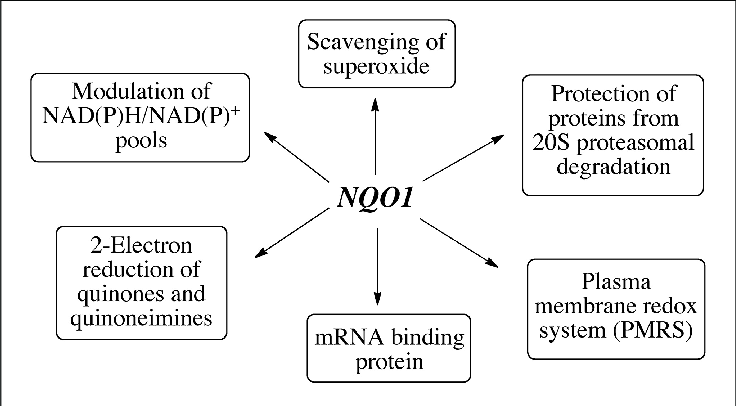
The NQO1 gene encodes the NAD(P)H: quinone oxidoreductase 1 enzyme, which plays a crucial role in the body’s phase II detoxification processes. This enzyme utilizes NADH or NADPH to convert quinones, which are reactive intermediates formed during the breakdown of various substances, into hydroquinones, making them easier for the body to eliminate.
In addition to quinones, NQO1 is also involved in the metabolism of benzene metabolites and a few specific chemotherapy drugs. NQO1 can also act as a direct antioxidant by scavenging superoxide.
If you think back to high school chemistry, you may remember redox reactions – where the reactants either lost or gained an electron. NQO1 acts as a reducing agent, which is important in the body’s ability to make certain substances easier to eliminate.
Quinones and hydroquinones:
The NQO1 gene codes for the enzyme NAD(P)H: quinone oxidoreductase 1 enzyme. This enzyme uses NADH or NADPH to reduce quinones to hydroquinone. Quinone is a general term that refers to a class of organic compounds with a certain structure. They are formed in the conversion of aromatic compounds, such as benzene or naphthalene.
Quinones are usually formed in the body as intermediates – the product that occurs when the body is breaking down certain substances. These types of reactions go on all the time in the body. Because quinones are so reactive, it is important for the body to convert quinones into hydroquinones quickly.
For example, when the body breaks down estrogen for elimination, an intermediate is formed, which is an estrogen quinone metabolite linked to breast cancer risk. NQO1 can help to metabolize and get rid of estrogen quinone metabolites, thus decreasing cancer risk.
Related article: Estrogen Metabolism Genes
NQO1 is also involved in cellular defense against oxidative stress, as well as the conversion of CoQ10 and the conversion of vitamin K. In the reduction (chemistry meaning, think redox reaction) of Vitamin K, NQO1 is mostly involved in turning vitamin K3 into an active form for blood clotting and bone-building[ref].
Related article: Vitamin K genes
NQO1 is also involved in breaking down outside toxins such as benzene and some chemotherapy drugs. Benzene is a carcinogen found in gasoline fumes, laundry detergent, furniture wax, industrial uses, pesticides, and smoke. Benzene is also a component of smog. NQO1 comes into play in the phase II metabolism of benzene after it has been acted on by CYP2E1. [ref][ref]
Related article: CYP2E1 gene
Additionally, NQO1 is important in the process that a cell goes through to divide and create a new cell. NQO1 has been shown to interact with NAD+ and SIRT2 during cell division.[ref]
Related article: NAD+, NR, NMN and genetics
Link to gut health:
Animal studies show that a non-functioning NQO1 gene leads to gut impermeability and inflammation (leaky gut)[ref].
NQO1 in the brain:
NQO1 is important in moderating the inflammatory response in the brain in neuroinflammation. Acting as an antioxidant, NQO1 can reduce oxidative stress in the brain. Essentially, NQO1 is reducing quinones in a way that prevents the formation of oxidative stress. Quinones can easily undergo redox reactions, and one type of reaction forms semiquinone radicals, which are reactive oxygen species that cause oxidative stress. NQO1 converts quinones in a way that avoids making semiquinone and instead skips to hydroquinone, which is water-soluble and able to be excreted.[ref]
One way that NQO1 protects the brain from ROS is in the interaction with vitamin E, also called tocopherol. The α-tocopherol quinone is formed in reactions where vitamin E is acting as an antioxidant. That quinone then can be converted to the hydroquinone form, preventing oxidative stress in the cell.
Genetic variants in the NQO1 gene (see genotype report below) increase the risk for Parkinson’s disease, and decreased NQO1 function is linked to an increased risk of Alzheimer’s disease.[ref]
NQO1 and Nrf2 pathway:
Nrf2 (Nuclear factor erythroid 2-related factor 2) is a transcription factor that regulates the expression of antioxidant and detoxification genes, including NQO1. The activation of the Nrf2 pathway can lead to increased NQO1 expression, enhancing the body’s defense against oxidative stress and xenobiotics.
Related article: Nrf2 pathway
NQO1 Genotype Report:
Research shows there are two common NQO1 variants that reduce function and increase the risk for certain cancers. The variant NQO1*2 (P187S) leads to a deficiency in the enzyme, while the NQO1*3 (R139W) variant has reduced enzyme activity, which may be dependent on the substance.
Members: Log in to see your data below.
Not a member? Join here.
Why is this section is now only for members? Here’s why…
Member Content:
Why join Genetic Lifehacks?
~ Membership supports Genetic Lifehack's goal of explaining the latest health and genetics research.
~ It gives you access to the full article, including the Genotype and Lifehacks sections.
~ You'll see your genetic data in the articles and reports.
Join Here
Lifehacks:
Avoiding toxins:
Poor NQO1 function is a problem in conjunction with aromatic compounds that are detoxified in phase II metabolism to form quinones.
Benzene and naphthalene are two of these compounds. Benzene is found in smog, petroleum products, cigarette smoke, and various industrial chemicals.
Naphthalene has a pungent odor and is found in mothballs. While I am not finding any specific studies on NQO1 polymorphisms and mothballs, if you have reduced NQO1 function, you may want to avoid breathing in naphthalene.
Melatonin:
More than just a sleep hormone, melatonin has many roles in cellular health. A study on smokers found that melatonin supplementation (3g/night) increased Nrf2 and NQO1 levels and reduced oxidative stress.[ref]
A natural way to boost your melatonin levels at night is to avoid blue light, such as from LED lights and screens, for a couple of hours before bedtime. Exposure to full sun during the morning hours also boosts melatonin at night.
Natural Supplements with research for boosting NQO1:
What can you do if you have a poor NQO1 function? There are several natural substances that may boost the function.
Member Content:
Why join Genetic Lifehacks?
~ Membership supports Genetic Lifehack's goal of explaining the latest health and genetics research.
~ It gives you access to the full article, including the Genotype and Lifehacks sections.
~ You'll see your genetic data in the articles and reports.
Join Here

