Key takeaways:
- AHCC is a shiitake mushroom extract that acts as an immune modulator, boosting NK cells, T cells, and dendritic cells.
- Clinical trials show benefits for HPV clearance and reducing side effects from chemotherapy.
- AHCC induces the CYP2D6 enzyme, which could affect the metabolism of roughly 25% of prescription drugs.
- Most studies are small, preliminary, or industry-funded — larger independent trials are still needed.
AHCC & Immune Regulation:
Mushrooms and mushroom extracts have long been used for medicinal purposes, and AHCC mushroom has been the subject of many modern clinical studies.
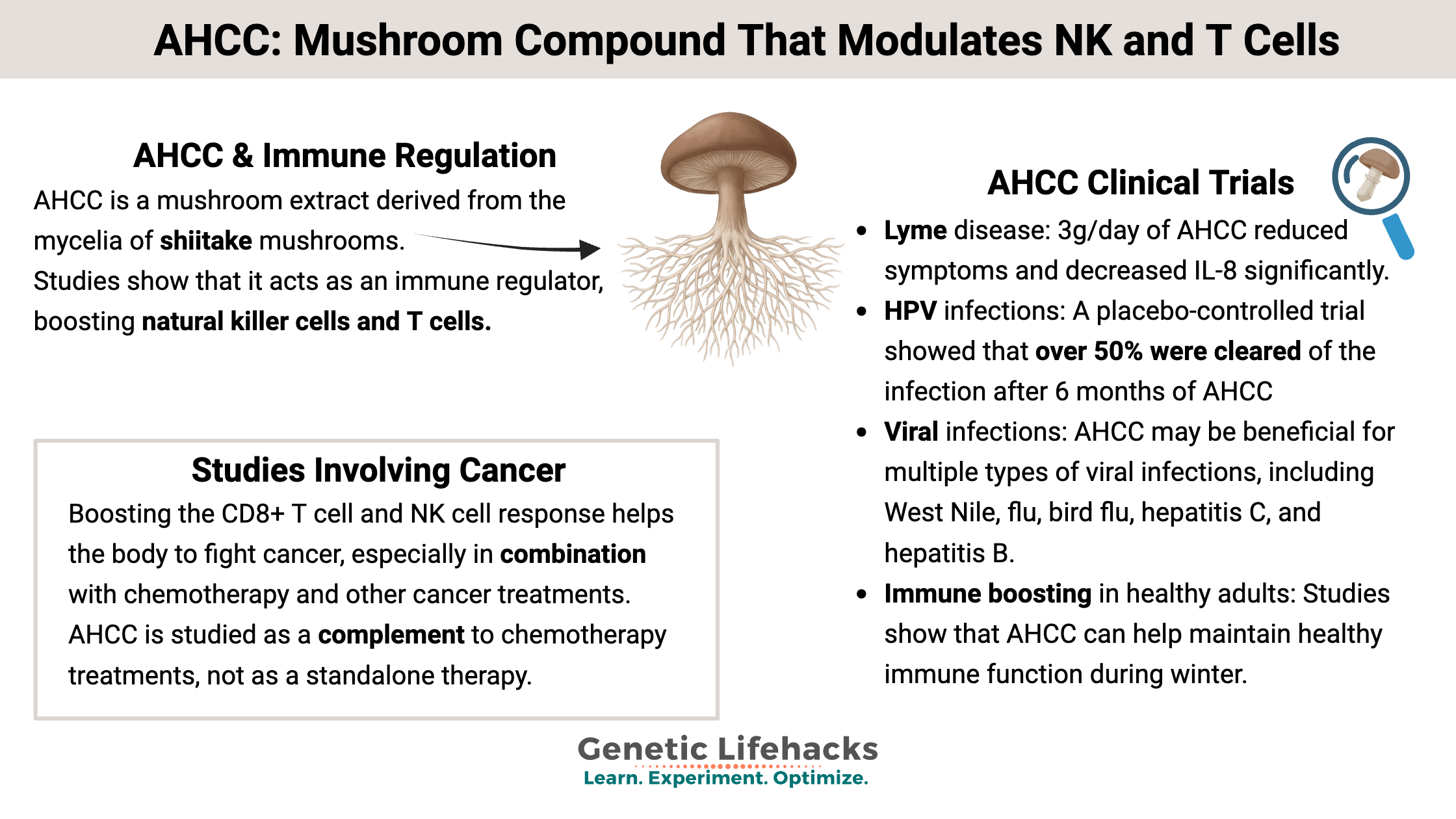
AHCC, which stands for active hexose correlated compound, is a mushroom extract derived from the mycelia of shiitake mushrooms (Lentinus edodes). It is a proprietary compound from Japan produced by fermenting the shiitake mycelium to increase alpha-1,4-glucan oligosaccharides. Studies show that it acts as an immune regulator, boosting natural killer cells and T cells.
The AHCC compound is made through a liquid culture process that allows the mycelia of the shiitake mushroom to proliferate and produce fungal bodies, but not fruiting bodies (not the mushrooms we typically think of eating). The compound is then separated from the cultured mycelia and freeze-dried. This is a patented process by a Japanese company called Amino Up Co, Ltd who has been researching and developing it since 1987.[ref]
What is AHCC used for? Examining the studies and trials.
When reading studies on AHCC, note that many are sponsored, at least in part, by the compound’s manufacturer, Amino Up. This doesn’t mean that the studies are automatically invalid, but just that they should be read more closely.
In general, the studies on AHCC show that it is an immune modulator – it can both upregulate and downregulate immune activity depending on context.
- Innate immune system: Boosts NK (natural killer) cells, dendritic cells, and macrophages
- Adaptive immunity: Increases CD4+ and CD8+ T lymphocytes; modulates interferon pathways (IFN-β and IFN-γ)
HPV infections:
A placebo-controlled, crossover trial in women over age 30 with chronic HPV showed that over 50% were cleared of the infection after 6 months of AHCC (3g/day on an empty stomach). This phase II study involved women with confirmed persistent high-risk HPV infections for more than 2 years. The AHCC suppressed IFN-β, which stimulated an increase in T lymphocytes and IFN-γ, leading to the clearance of the HPV infection.[ref]
Lyme disease:
A small open-label trial in patients with chronic Lyme disease showed that after 8 weeks of 3g/day of AHCC, the patients had reduced symptoms (“ameliorated flu-like symptoms and manifestations in the eye, joint, muscle, nervous system and cardiovascular system.”) The researchers also measured inflammation markers and found that IL-8 was significantly decreased.[ref]
Related article: Chronic Lyme Disease
Viral infections:
Studies on AHCC show that it may be beneficial for multiple types of viral infections, including West Nile, flu, bird flu, hepatitis C, hepatitis B, and herpes.[ref]
Numerous studies in animals with viral infections show that AHCC increases natural killer cell activity, which then increases the body’s natural immune response to clear the viruses.[ref]
Immune boosting in healthy adults:
A 2015 study examined whether AHCC supplementation could maintain immune function during the seasonal immune decline associated with winter. The research involved 34 healthy volunteers who took either 1.0 g/day of AHCC or a placebo for 4 weeks during early winter. Normally, there is a seasonal decline in immune response during this time, which was seen in the placebo arm of the trial. In the group taking AHCC, they preserved their natural killer (NK) cell counts despite seasonal changes. Overall, the immune competence score stayed stable in the AHCC group but dropped in the placebo group during winter.[ref]
Related article: T cell exhaustion
In a separate trial, researchers also investigated antibody response to the flu vaccine. This small clinical trial used 3g/day AHCC compared to a placebo arm, with the supplement started immediately after getting the flu shot. The results showed that the AHCC group had increased NK response and increased CD 8+ T cell response. Antibody titers were also higher in the AHCC group.[ref]
A clinical trial involving 40 healthy adult male volunteers tested AHCC, Bifidobacterium longum, or a placebo to see the effect on immune response. The study showed that the group receiving the combination of AHCC plus Bifidobacterium longum had an increase in myeloid dendritic cell counts, which supports an anti-inflammatory immune profile. [ref]
Studies involving cancer:
Boosting the CD8+ T cell and NK cell response helps the body to fight cancer, especially in combination with chemotherapy and other cancer treatments. AHCC is studied as a complement to chemotherapy treatments, not as a standalone therapy.[ref]
In addition to the effect on T cells and NK cells, studies involving colon cancer note that the glucans in AHCC also increase microbial diversity in the gut in a beneficial way. [ref]
A cell line study using cancer stem cells showed that AHCC alters microRNA expression. It upregulated miR-355, which is known to be beneficial for tumor suppression.[ref]
Related article: MicroRNAs
Animal studies show that AHCC may have antitumor activity against melanoma and also may potentiate the effects of 5-fluorouracil in liver tumors.[ref][ref]
The human clinical trials of AHCC show that it reduces chemotherapy-related adverse events.
- In pancreatic cancer patients, a clinical trial showed that 6g/day of AHCC reduced the frequency of side effects, including taste disorders, caused by the chemo.[ref]
- Another study showed that AHCC improved the quality of life scores of patients undergoing chemotherapy for various cancers.[ref]
A study involving patients who had liver resection for hepatocellular carcinoma showed that 3g/day for two years was safe and improved inflammation levels. However, the small study didn’t show statistically significant improvement in preventing recurrence, compared to the normal recurrence rate.[ref]
Safety and precautions:
Talk with your doctor if you have questions about AHCC or any supplement.
With the immune-stimulating effects, people with autoimmune conditions and anyone on immunosuppressant drugs should use caution or consult their doctor.
A phase I safety trial in healthy adults showed that 9 g/day of AHCC for 14 days caused no blood lab work abnormalities, but at that level, some of the trial participants had nausea, diarrhea, bloating, headache, and fatigue. Note that this is a much higher dose than is used in other clinical trials (usually 3g/day).[ref]
Drug interactions: AHCC induces CYP2D6, which is the enzyme used for metabolizing about 25% of prescription medications. It also induces aromatase and may reduce the activity of aromatase inhibitor drugs such as letrozole.[ref][ref]
Genetic connections:
There are no studies that look at AHCC efficacy along with genetic variants. Instead, this section brings in genetic variants that may interact with AHCC in specific circumstances.
CYP2D6 gene
If you have genetically altered CYP2D6 function, it may be particularly important to avoid taking AHCC along with medications metabolized by CYP2D6.
| Gene | RS ID | Your Genotype | Effect Allele | Effect Allele Frequency | Notes About Effect Allele |
|---|---|---|---|---|---|
| CYP2D6 | rs3892097 | -- | T | 0.17 | CYP2D6*4 decreased function |
| CYP2D6 | rs5030655 | -- | D | 0.001 | CYP2D6*6 deletion; decreased function |
| CYP2D6 | rs5030656 | -- | D | 0.01 | CYP2D6*9 decreased function |
| CYP2D6 | rs1065852 | -- | A | 0.21 | CYP2D6*10 decreased function |
| CYP2D6 | rs28371725 | -- | T | 0.09 | CYP2D6*41 decreased function |
| CYP2D6 | rs1135824 | -- | C | 0.0007 | CYP2D6*3 decreased function |
| CYP2D6 | rs5030867 | -- | G | 0.0005 | CYP2D6*7 decreased function |
| CYP2D6 | rs28371706 | -- | A | 0.01 | Possibly decreased function |
| CYP2D6 | rs16947 | -- | A | 0.32 | CYP2D6*2 variant; possibly reduced function |
| CYP2D6 | rs1135840 | -- | G | 0.57 | G/G only: higher response rate to propranolol; adverse liver events with antituberculosis drugs[ |
T Cell Exhaustion
For someone who has T cell checkpoint genetic variants and is under an immune system strain (e.g. immunotherapy, chronic viral or bacterial infection, long Covid), AHCC may be worth investigating further and talking with your doctor about.
| Gene | RS ID | Your Genotype | Effect Allele | Effect Allele Frequency | Notes About Effect Allele |
|---|---|---|---|---|---|
| TIM3 | rs13170556 | -- | C | 0.14 | increased susceptibility to T cell exhaustion, which increases risk of chronic lung infections |
| PDCD1 | rs2227981 | -- | A | 0.42 | Lower PDCD1 expression, theoretically less likely to have T cell exhaustion; reduced cancer risk |
| PDCD1 | rs10204525 | -- | T | 0.15 | Likely higher PDCD1 expression, more likely to have T cell exhaustion; increased cancer risk |
| CTLA4 | rs231775 | -- | G | 0.38 | decreased risk of T cell exhaustion in cancer therapy (greater survival rates); increased risk of autoimmune disease |
| LAG3 | rs3782735 | -- | G | 0.39 | increased survival in gastric cancer (likely lower LAG3, less T cell exhaustion) |
| LRRC8C | rs10493829 | -- | C | 0.41 | elevated LRRC8C, improved survival in certain cancers |
| HLA-DRB1 | rs660895 | -- | G | 0.21 | lower risk of T cell exhaustion; higher risk of rheumatoid arthritis (~6-fold) and autoimmune diseases (tag for HLA-DRB1*0401) |
| PDCD1 | rs2227982 | -- | A | 0.03 | higher PDCD1 expression; more likely to be a non-responder to chemotherapy for cervical cancer |
| CTLA4 | rs3087243 | -- | A | 0.43 | higher CTLA4 levels; more likely to be a non-responder to chemotherapy for cervical cancer |
IL-15 and NK cells
Similar to the T cell exhaustion variants, genetic variants in IL-15 can affect both NK cells and T cells. For someone under an immune system strain with variants below, AHCC may help with improving NK response when the immune system is under strain.
| Gene | RS ID | Your Genotype | Effect Allele | Effect Allele Frequency | Notes About Effect Allele |
|---|---|---|---|---|---|
| IL15 | rs10833 | -- | T | .33 | T/T: lower IL15; increased liver fibrosis in hepatitis C, but better response to treatment; increased risk of subclinical atherosclerosis; C/T: lower IL15 and increased risk of subclinical atherosclerosis |
| IL15 | rs2857261 | -- | A | .52 | A/A: Increased risk of celiac disease; A/G: no increased risk of celiac |
| IL15 | rs17007695 | -- | C | .08 | increased relative risk of acute lymphoblastic leukemia |
| IL-15RA | rs2228059 | -- | G | .5 | increased strength gains in resistance training; decreased relative risk of esophageal or gastric cancer |
| IL-15RA | rs7097780 | -- | G | .32 | increased risk of symptomatic osteoarthritis |
Limitations and what is still lacking:
I want to emphasize here that there are limitations on what we know about this specific mushroom compound. Here are some of the noted limitations:
- Many studies are small-scale, preliminary, or industry-funded (Amino Up)
- Most cancer data comes from animal models or retrospective human studies
- Lack of large, independent randomized controlled trials for most applications
- No standardized treatment protocols or FDA-approved indications
Overall, the studies on AHCC are encouraging, especially for immune support and for HPV. The cancer studies are small and preliminary, though, and more clinical trials are needed.
Related articles:
Nicotinamide Riboside and NMN: Boosting NAD+ in Aging with Supplements
Cordyceps: Clinical Trials, Mechanism of Action, and Genetic Connections
References: