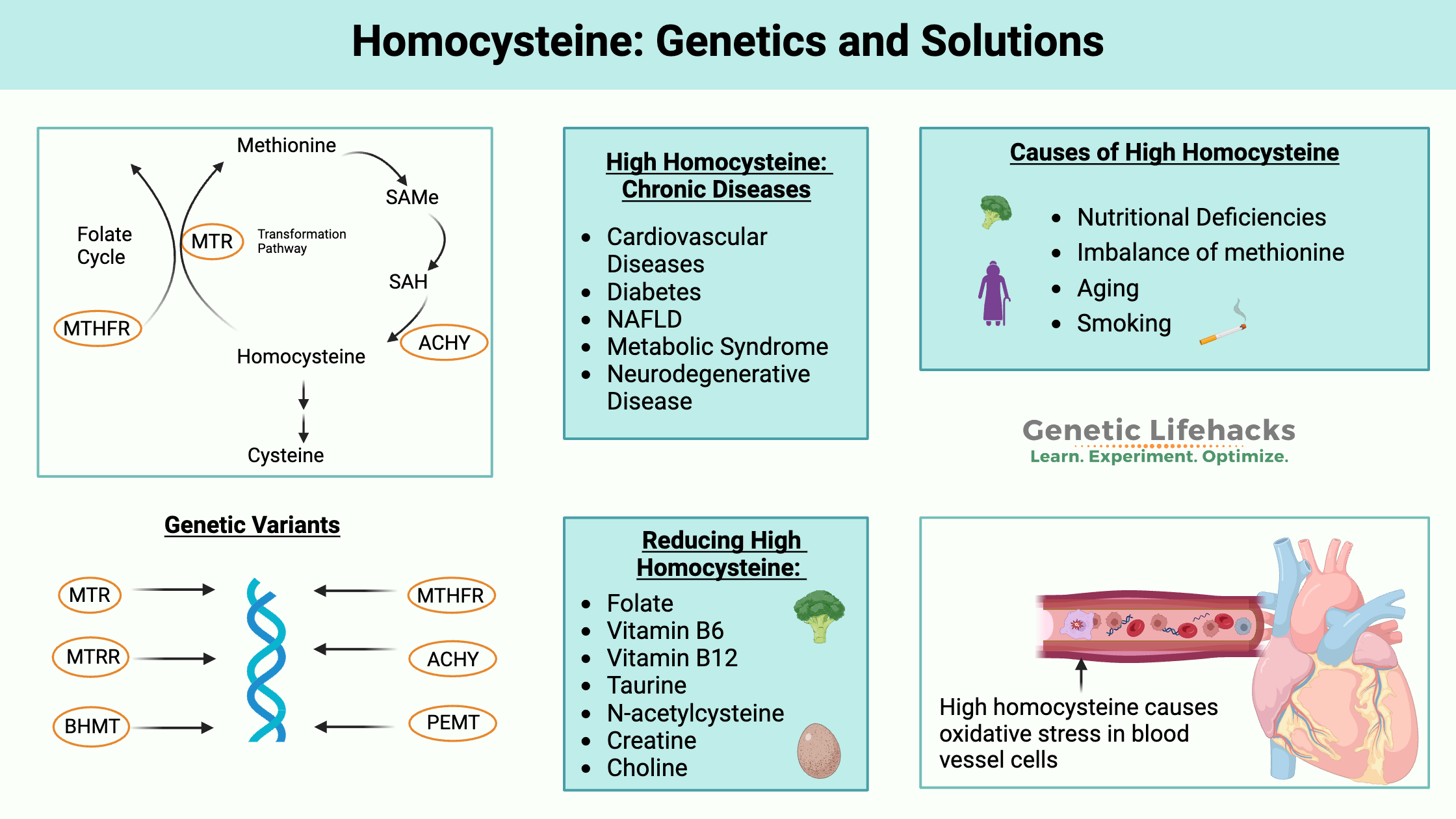
Key takeaways:
~ Research shows that high homocysteine levels can cause excess oxidative stress and endoplasmic reticulum stress.
~ Epidemiologic studies show that high homocysteine levels are strongly linked to an increased relative risk of cardiovascular diseases.
~ Homocysteine is an intermediate produced in the methionine cycle and can be remethylated to methionine or converted to cysteine.
~ Genetic variants in several pathways interact with what you eat (or don’t eat) to increase homocysteine levels.
Let’s dig into how homocysteine is formed and what high homocysteine can mean for your health.
Preview your genes:
| Gene | RS ID | Effect Allele | Your Genotype | Notes About Effect Allele |
|---|---|---|---|---|
| MTHFR | rs1801133 | A | -- | MTHFR C677T, higher homocysteine levels, especially if folate is lacking |
| NOX4 | rs11018628 | C | -- | decreased homocysteine, decreased stroke risk |
| MTR | rs1805087 | G | -- | increased risk of cognitive impairment due to higher homocysteine |
| MTR | rs2275565 | T | -- | associated with higher homocysteine levels |
| MTRR | rs1801394 | G | -- | somewhat increased homocysteine levels, especially if riboflavin is low |
| CBS | rs5742905 | G | -- | risk of increased homocysteine, responsive to vitamin B6 |
| PON1 | rs662 | C | -- | higher homocysteine-thiolactone levels |
| PEMT | rs7946 | T | -- | TT: homocysteine increases with low folate diet |
| BHMT | rs3733890 | A | -- | reduced conversion of choline to betaine |
This article is free and open to everyone as an example of what a full Genetic Lifehacks article offers!Members: Be sure you are Logged in to see your genotype data below.
What is homocysteine?
Homocysteine is a sulfur-containing amino acid that is a byproduct of the metabolism of methionine.
What’s methionine? Methionine is an essential amino acid that we get from protein-rich foods. Essential means that it is an amino acid that we have to get from foods, and it is needed for the production of proteins in the body.
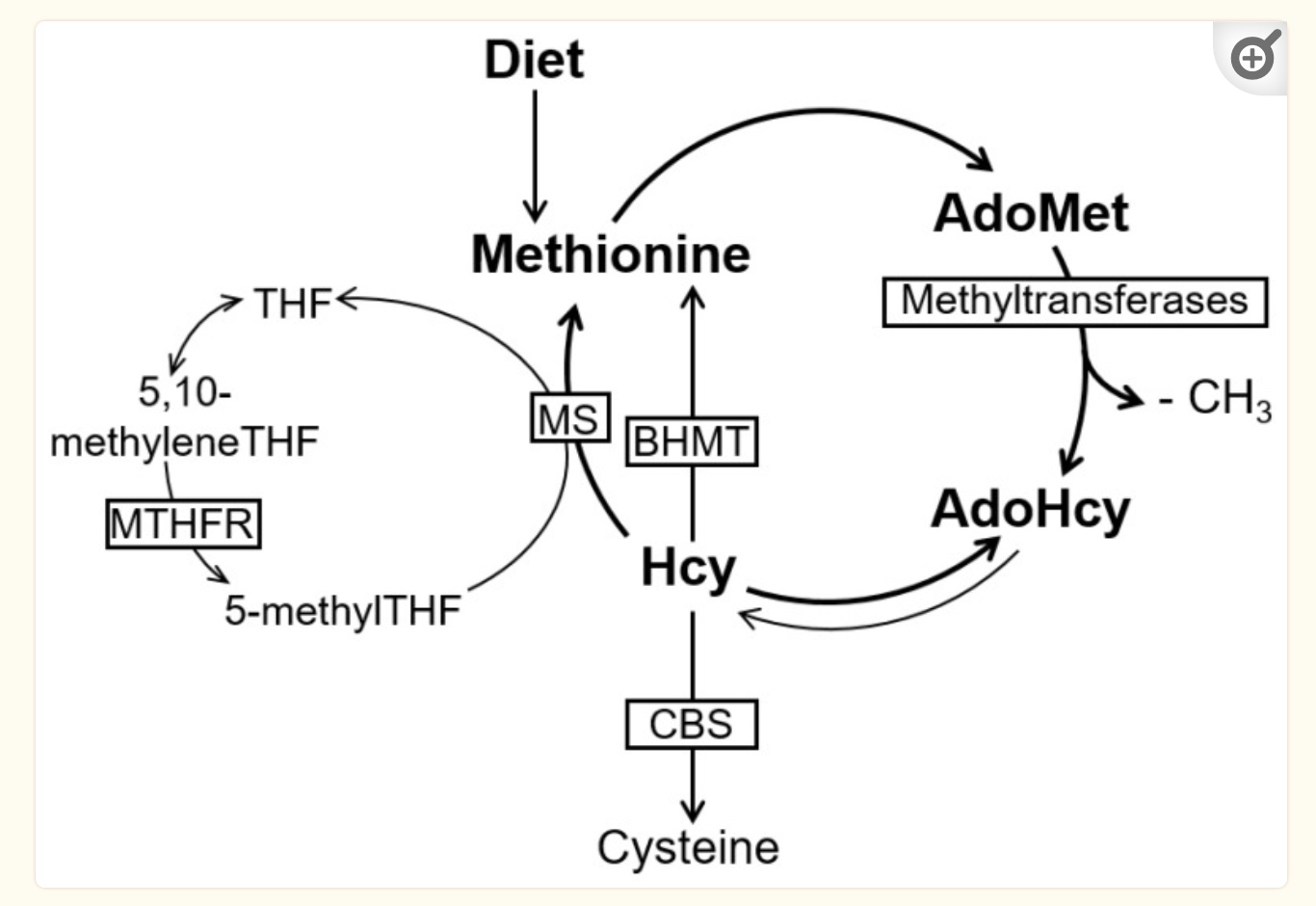
Methionine is used by cells to supply methyl groups, which are required in many different cellular reactions. In the methionine cycle, methionine receives adenosine from ATP, which then creates SAM-e (s-adenosyl-methionine). SAM-e can then transfer a methyl group for methylation reactions. When SAM-e loses a methyl group, it becomes SAH (s-adenosyl-homocysteine), which then is hydrolyzed to form homocysteine.[ref]
Homocysteine can then be converted back to methionine or transformed in other pathways.
Why are methyl groups so important? Methyl groups are used in cells in a variety of ways. They are essential in the conversion of serotonin to melatonin and the creation of creatine and phosphatidylcholine. Methyl groups are also used to make neurotransmitters, to silence genes, and to detoxify certain toxins.
When any of these pathways are blocked, homocysteine levels can rise, which causes negative long-term health effects.
How are homocysteine levels maintained?
Homocysteine is constantly being formed and converted. These are ongoing cycles that are constantly happening in your cells, and homocysteine levels are regulated by the cells to maintain a fairly consistent level.
The level of homocysteine depends on the amount that is formed in the conversion of SAMe and SAH (S-adenosyl homocysteine) and the amount that is recycled or further broken down.
In cells, homocysteine is metabolized in three ways:[ref]
- It can be remethylated to form methionine (requires folate and vitamin B12 – or needs betaine, BHMT gene)
- Can be converted to cysteine (serine and B6 as a cofactor), which is used in the synthesis of glutathione
- It can be converted to homocysteine thiolactone, which can be a problem at higher levels
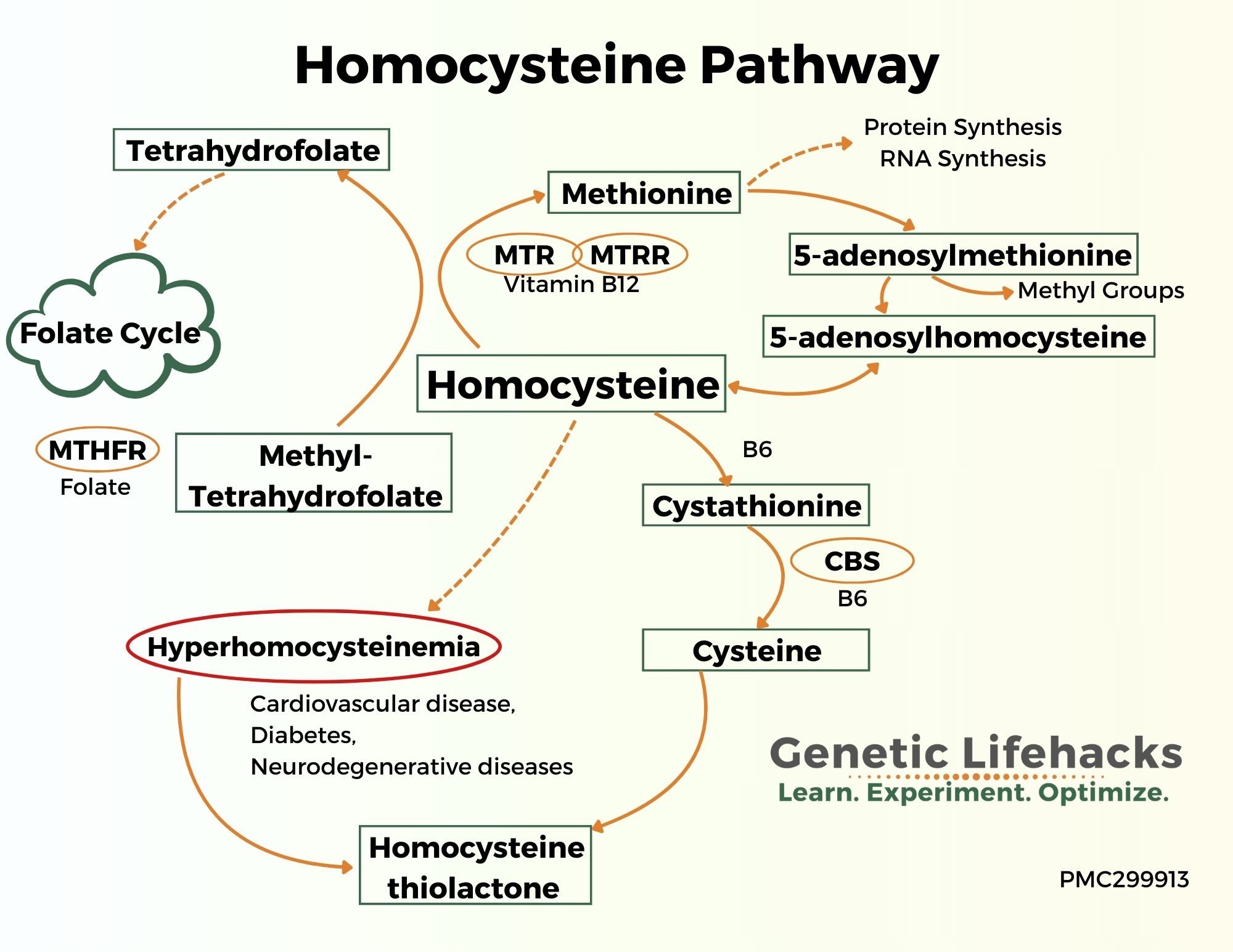
Let’s dig into each of these pathways further:
Methionine Cycle: Homocysteine, Methionine, SAMe and SAH
Homocysteine is maintained at a relatively constant level by several mechanisms in the methionine cycle.
Most homocysteine is remethylated to methionine using methyl groups from the folate cycle or donated from trimethylglycine (also called betaine). This process occurs primarily in the liver and requires methylfolate along with vitamin B12.
When SAH is high, methylation reactions are decreased, reducing the conversion of methionine to SAMe and methyl groups. When SAMe is low, the remethylation of homocysteine to methionine is prioritized in the cell (instead of the transsulfuration pathway).[ref]
Many methyl groups are used by cells to make creatine, so when creatine levels are higher, it can reduce the need for methyl groups. Another major use of methyl groups in the body is for the synthesis of phosphatidylcholine from choline, so when phosphatidylcholine levels are higher, there is less of a strain on the methylation cycle.[ref]
Homocysteine goes down the transsulfuration pathway to form cysteine:
Another path that homocysteine can take is to be converted to cysteine through a two-step process in the transsulfuration pathway. This occurs primarily in the liver and kidneys. Vitamin B6 is required as a cofactor, and serine is also used. The CBS enzyme catalyzes the reaction.
Related article: CBS gene and sulfur
Cysteine is a sulfur-containing amino acid that can be converted to taurine or sulfates, including glutathione. Glutathione is an antioxidant used in cells to counteract oxidative stress.
The flux of cysteine and the end products are driven by how much methionine is eaten as well as how much glutathione is needed to combat oxidative stress in cells.[ref][ref]
Homocysteine thiolactone:
Homocysteine can also become homocysteine thiolactone when a thiol group is attached. While the methylation cycle gets a lot of press, the metabolism of homocysteine-thiolactone is also really important.
Homocysteine thiolactone can cause damage to some types of cells, particularly the endothelial cells that line blood vessels. Therefore, the body has ways to get rid of homocysteine-thiolactone quickly. Most homocysteine thiolactone is eliminated through the kidneys, so good kidney function is important.
PON1, an enzyme that circulates in the blood attached to HDL cholesterol, metabolizes homocysteine-thiolactone back to homocysteine in the bloodstream. PON1 levels vary widely among individuals, in part due to genetic variants in PON1. Some of the negative effects of high homocysteine levels are modulated by the PON1 genotype.[ref]
Let’s take a look at why high homocysteine levels cause problems in the body, and then we’ll look at genetic variants in the above cycles that can affect homocysteine levels when combined with dietary choices.
Why is high homocysteine a potential problem?
Epidemiological studies show that high homocysteine levels correlate with an increased incidence of blood clots, heart attacks, and strokes.
The question has been whether high homocysteine causes these conditions or whether it is simply a marker of cellular pathways that were altered in these conditions.
Recent research gives us insight into how and why high homocysteine levels cause adverse effects on the cardiovascular system and long-term health.
Post-translational protein modifications:
Recent research shows that homocysteine can bind to proteins after they are synthesized in post-translational protein modification. Proteins are made up of amino acids that are arranged in a specific way. The incorporation of homocysteine (an amino acid) ultimately changes the structure of the protein and can affect its function.
For example, higher levels of homocysteine mean that certain proteins important in cardiovascular disease and neurodegenerative diseases can end up with a homocysteine attached or incorporated into them.[ref]
The process of post-translational protein modification happens all the time and in a number of different ways, including the addition of other amino acids, sugar molecules, or fatty acids.
The problem arises when too much homocysteine is available and ends up being incorporated into too many proteins. For example, homocysteine may be incorporated into certain proteins instead of methionine. The addition of homocysteine causes some proteins to become pro-inflammatory or pro-thrombotic.[ref]
Increased oxidative stress and ER stress due to high homocysteine:
High homocysteine levels are also associated with increased oxidative stress and endoplasmic reticulum (ER) stress.
Animal studies show that increasing methionine in the diet slightly increases homocysteine levels. This slight increase then leads to an increase in inflammatory cytokines (TNF-alpha, IL-1B) in macrophages[ref]
The endoplasmic reticulum (ER) is an organelle in cells where newly synthesized proteins are folded, lipids are synthesized, and calcium is stored.
Proteins must be properly folded – have the right shape – to function properly in a cell. Within the ER (endoplasmic reticulum), there are processes that check to make sure that proteins are made and folded correctly. If the proteins are not correct or there is a stressor that causes too many proteins to be needed, a response pathway called the unfolded protein response kicks in. When overwhelmed, this response promotes cell death. This entire process prevents the cell from producing misfolded or altered proteins.[ref]
Studies show that high homocysteine levels cause ER stress and activate the unfolded protein response pathway, which can lead to cell death. High homocysteine can induce oxidative stress in the ER. It can also modify proteins by incorporating homocysteine instead of methionine into the proteins. This change in sulfide bond formation then causes protein misfolding.[ref]
High homocysteine and chronic diseases:
High homocysteine can cause chronic health conditions by:
- Increasing oxidative stress
- Causing endoplasmic reticulum (ER) stress
- Incorporation into proteins in the place of methionine
Mendelian randomization studies can be used to show a cause-and-effect relationship between a marker, such as homocysteine, and a disease.
Diabetes:
Type 2 diabetes is associated with higher homocysteine levels, and genetic Mendelian randomization studies show that higher homocysteine is a causative factor. Researchers believe this is due to increased ROS (increased oxidative stress) in the pancreatic beta cells.[ref]
Fatty liver disease:
High homocysteine levels are associated with NAFLD (fatty liver disease). A recent analysis using Mendelian randomization showed that genetically predicted homocysteine levels increased the odds of NAFLD.[ref]
Cardiovascular disease:
To answer the question of whether “a modestly elevated homocysteine level is causally associated with an increased risk of cardiovascular disease”, researchers again used huge genetic data sets and Mendelian randomization to determine causality. Modestly higher homocysteine as predicted by genetics was associated with an increased risk of stroke.[ref]
Chronic diseases:
Similarly, MR studies have shown that high homocysteine can cause metabolic syndrome, worsen osteoarthritis, increase the risk of cataracts, and increase the risk of Alzheimer’s disease. [ref][ref][ref][ref]
In addition, lowering homocysteine levels by supplementing with B vitamins has been shown to reduce the rate of brain atrophy in elderly people with mild cognitive impairment.[ref] Researchers have found that high homocysteine specifically exacerbates amyloid-beta-induced cell death in the brain.[ref]
Researchers think the connection to high homocysteine for osteoporosis could be due to dysregulation of the transsulfuration pathway rather than a problem with the remethylation of homocysteine. The dysregulation of the transsulfuration pathway could result in higher homocysteine levels and low cysteine and taurine levels.[ref]
How is high homocysteine defined?
The range of normal homocysteine levels is defined in some research studies as 5-15 μmol/L. Others use 13μmol/L as the upper limit of normal. Hyperhomocysteinemia (high homocysteine) is usually defined as greater than 15 μmol/L. [ref]
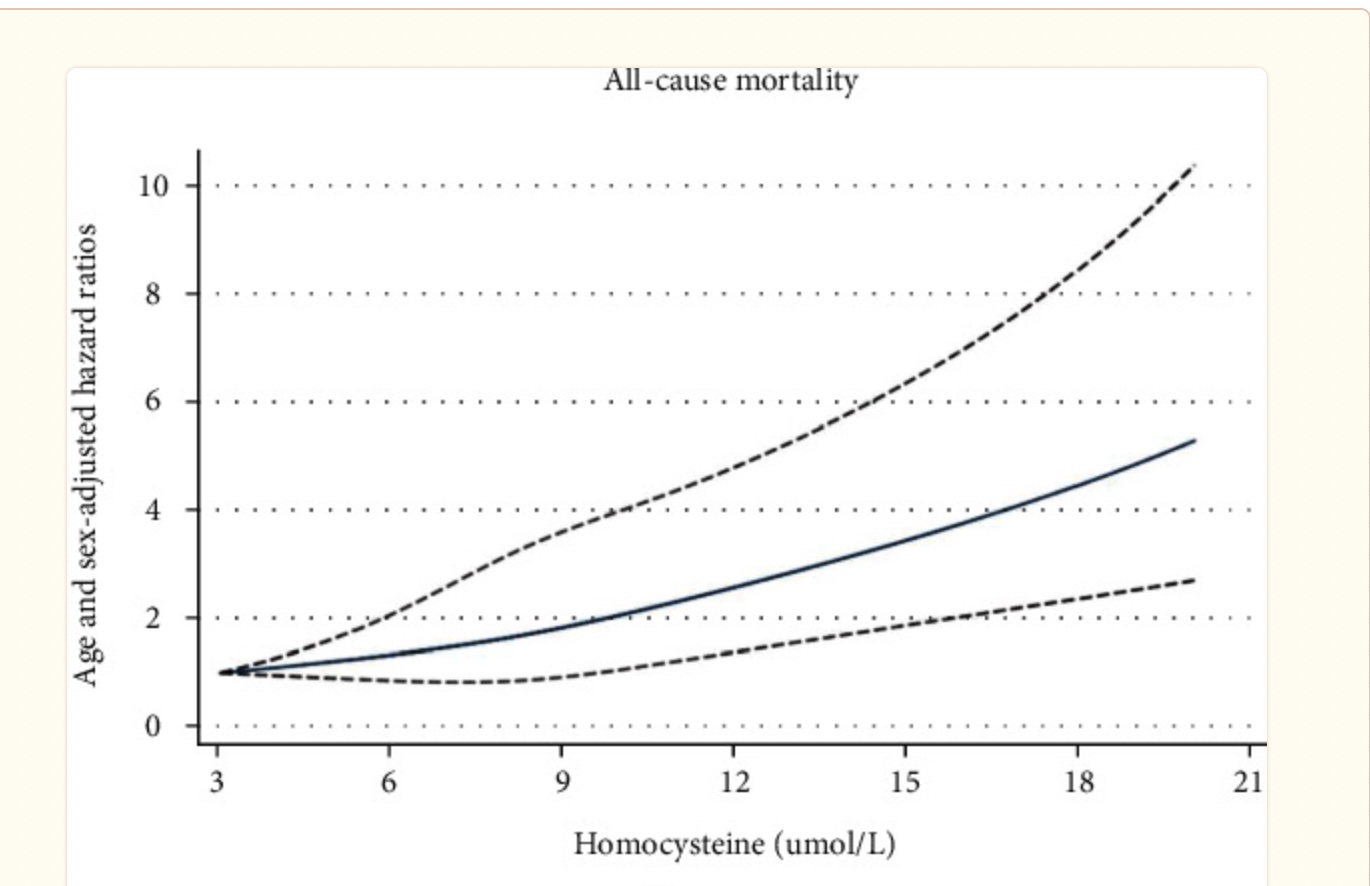
What is the optimal level for homocysteine?
This is a more difficult question to answer. Estimates of all-cause mortality seem to start rising around homocysteine levels of 9-10 μmol/L, but there is likely a lot of individual variation in whether homocysteine below the upper limit of 13-15 μmol/L is worrisome.
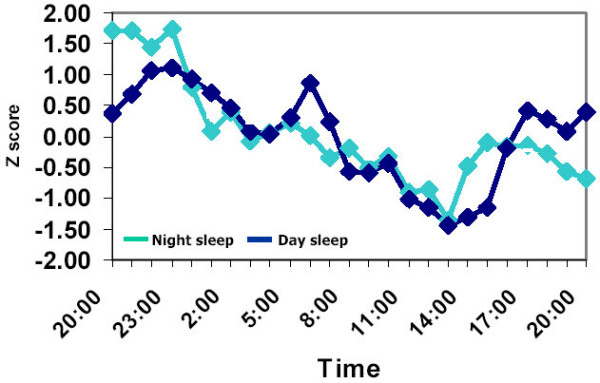
Circadian rhythm of homocysteine:
A study in healthy adults showed that homocysteine levels vary ~ 3μmol/L over the course of a day. The peak is around midnight and the lowest levels are around 2 pm. The study also showed that eating a meal with a lot of protein (containing the amino acid methionine) increases homocysteine levels a little bit over the next 8 hours.[ref]
Two important takeaways here:
- If you are tracking your homocysteine levels with blood tests, get the tests done at the same time of day. An afternoon test is likely to give you a test result that is several points lower than an early morning test.
- Similarly, eating a high-protein meal several hours before the test will raise homocysteine levels a little bit.
What raises homocysteine levels?
Homocysteine levels can be high for a number of reasons:[ref][ref]
- Insufficient folate and B12 (exacerbated by variants in MTHFR, MTR)
- Not enough B6 (CBS pathway)
- More methionine than the body can handle
- Renal insufficiency (kidney disease) or alcoholism
Genetic mutations in the CBS gene can cause rare cases of hyperhomocysteinemia. Children with rare mutations in CBS or MTR genes can have neural tube defects, early atherosclerosis, or neurological effects.[ref]
Related article: MTHFR and the methylation cycle
Homocysteine levels, sex, and age:
Men tend to have higher homocysteine levels than women, and homocysteine levels in both sexes tend to increase slightly with age. Smoking is also associated with higher homocysteine levels.[ref][ref][ref]
Folate and B12 status:
In general, higher folate and vitamin B12 levels are associated with lower homocysteine levels.[ref] This association holds true with aging.[ref]
Amount of methionine in the diet:
Methionine is an essential amino acid found in protein-rich foods. While we need methionine and protein in the diet, one way to increase homocysteine is to significantly increase dietary methionine without a concomitant increase in choline, B12, or folate.[ref]
Timing of testing:
Homocysteine levels are lowest in the first hour after a meal, so if you test your homocysteine level after eating, it will likely be different than a fasting blood test will show.[ref]
Down syndrome, CBS, and homocysteine:
People with Down syndrome usually have very low homocysteine levels due to the extra copy of chromosome 21, which causes an extra copy of the CBS gene. The extra CBS enzyme causes homocysteine to be lost through the transulfation pathway.[ref]
Homocysteine Genotype report:
MTHFR gene: encodes the enzyme needed for turning dietary folate into methylfolate, which is used as a methyl donor to remethylate homocysteine to methionine.
Check your genetic data for rs1801133 C677T (23andMe v4, v5; AncestryDNA):
- G/G: typical *
- A/G: one copy of MTHFR C677T allele, enzyme function decreased by 40%;
- A/A: two copies of MTHFR C677T, enzyme function decreased by 70 – 80%; higher homocysteine levels, especially if folate is lacking in the diet[ref][ref][ref]
Members: Your genotype for rs1801133 is —.
NOX4 gene: encodes an enzyme that increases the formation of ROS (reactive oxygen species). Knocking out NOX4 in mice causes lower homocysteine levels and lower cysteine and glutathione levels. Instead, the homocysteine is pushed through the betaine-dependent methylation pathway to be remethylated.[ref]
Check your genetic data for rs11018628 (23andMe v4, v5; AncestryDNA):
- T/T: typical
- C/T: decreased homocysteine, decreased stroke risk
- C/C: decreased homocysteine, decreased stroke risk[ref][ref] possibly lower glutathione levels
Members: Your genotype for rs11018628 is —.
MTR gene: encodes methionine synthase, an enzyme that utilizes vitamin B12 in the methionine cycle and thus needs plenty of available methylB12
Check your genetic data for rs1805087 A2756G (23andMe v4, v5; AncestryDNA):
- A/A: typical
- A/G: increased enzyme activity
- G/G: increased enzyme activity[ref], increased risk of cognitive impairment due to higher homocysteine[ref]
Members: Your genotype for rs1805087 is —.
Check your genetic data for rs2275565 (23andMe v4, v5; AncestryDNA):
- T/T: associated with higher homocysteine levels[ref]
- G/T: associated with higher homocysteine levels
- G/G: typical
Members: Your genotype for rs2275565 is —.
MTRR gene: Methionine synthase reductase (MTRR) encodes the enzyme that regenerates vitamin B12 (methylcobalamin) for use by MTR in the methionine cycle.
Check your genetic data for rs1801394 A66G (23andMe v4, v5; AncestryDNA):
- A/A: typical
- A/G: somewhat decreased enzyme efficiency; somewhat increased homocysteine levels, especially if riboflavin is low
- G/G: decreased enzyme efficiency [ref][ref][ref][ref][ref] somewhat increased homocysteine levels, especially if riboflavin is low[ref]
Members: Your genotype for rs1801394 is —.
CBS gene: encodes the cystathionine beta-synthase enzyme which acts with vitamin B6 in the transsulfuration pathway to remove homocysteine (forming cysteine, a building block of glutathione)
Check your genetic data for rs5742905 (23andMe v4 only):
- G/G: risk of increased homocysteine, responsive to vitamin B6[ref][ref]
- A/G: risk of increased homocysteine, responsive to vitamin B6
- A/A: typical
Members: Your genotype for rs5742905 is —.
PEMT gene: encodes phosphatidylethanolamine N-methyltransferase, an essential enzyme for the production of choline in the body. Using SAMe as a methyl donor, PEMT catalyzes the reaction to triple methylate PE to form phosphatidylcholine.[ref]
Check your genetic data for rs7946 V175M (23andMe v4, v5; AncestryDNA)
- C/C: typical PEMT activity (most common genotype worldwide)
- C/T: somewhat decreased PEMT enzyme activity
- T/T: decreased PEMT enzyme activity[ref] homocysteine increases with low folate diet[ref]( most common genotype for Caucasian populations)
Members: Your genotype for rs7946 is —.
BHMT gene: Encodes an enzyme needed for the conversion of dietary choline to betaine, which can be used to remethylate homocysteine into methionine.
Check your genetic data for rs3733890 R239Q G716A (23andMe v4, v5; AncestryDNA):
- A/A: reduced BHMT[ref][ref] reduced conversion of choline to betaine[ref] increased risk of early-onset heart disease with poor diet[ref]
- A/G: reduced BHMT, reduced conversion of choline to betaine, increased risk of early-onset heart disease with poor diet
- G/G: typical
Members: Your genotype for rs3733890 is —.
Lifehacks: Natural Ways to Lower Homocysteine Levels
The only way to know your homocysteine level is to get it tested. It’s an inexpensive test, and you can order it yourself in the US if you can’t get it done at your doctor’s office.
If your homocysteine level is high, the following dietary changes and supplements may help. As always, talk with your doctor if you have any medical questions or concerns about interactions between supplements and medications.
Diet changes for lowering homocysteine:
Folate-rich foods:
Increasing dietary folate by eating more folate-rich foods has been shown in clinical trials to lower homocysteine levels.[ref][ref][ref]
For example, one clinical trial found that adding more folate to the diet by increasing the number of servings of vegetables eaten each day reduced homocysteine levels by 13%.[ref]
Eating more folate-rich whole foods, such as dark leafy green vegetables, also has the benefit of increasing other vitamins and antioxidants.
Who will this work best for?
If you have the PEMT rs7946 TT genotype (above), a low-folate diet is more likely to raise your homocysteine levels. If you have high homocysteine with this genotype, you could focus on increasing dietary folate or supplementing with methylfolate.
For people with the MTHFR C677T homozygous variant (AA genotype above), adding more folate-rich foods significantly lowered homocysteine levels.[ref]
What if you get enough folate? Studies show a clear benefit for lowering homocysteine by raising folate to adequate levels, but there’s no additional benefit seen from adding more folate for people who already get plenty of folate.[ref][ref]
Decreasing protein may not be effective:
Higher levels of dietary methionine, such as from a high-protein diet, could theoretically increase homocysteine levels (this is how animal models of high homocysteine are created).
However, a high-protein diet usually contains more vitamin B12, vitamin B6, betaine, and folate, which may offset the increase in methionine in real life.[ref] In laboratory settings, people who eat foods high in methionine alone show an increase in homocysteine, but this is attenuated when the increased methionine is consumed along with other amino acids.[ref]
Natural vitamins and supplements for decreasing homocysteine:
Folate:
Dozens of studies and placebo-controlled clinical trials show that increasing folate can lower high homocysteine levels. Clinical trials use either folic acid (a synthetic formula that is converted into folate) or the active form of folate, methylfolate. Effective doses appear to start at 100 – 200 mcg/day.[ref][ref]
The RDA for folate from food is 400 mcg/day. For people with normal homocysteine levels (e.g., between 7-8), adding folic acid doesn’t statistically lower their homocysteine levels.[ref] When researchers compared adding more folate-rich foods (+350 mcg/day of folate) with a folic acid supplement (250 mcg/day of folic acid), they found that both groups had similar decreases in homocysteine.[ref]
Who will this work best for?
Anyone who is low in folate (and high in homocysteine) is likely to benefit from a folate supplement. People with the MTHFR C677T homozygous (AA genotype) have been shown to benefit most from folate to lower homocysteine. [ref]
Related article: Read about DHFR variants and folic acid
Vitamin B12:
Supplemental B12 may reduce high homocysteine levels in people who are deficient in intake.
There are several types of B12 available as supplements. People with slow COMT function (see below) may want to avoid supplementing with high doses of methyl donor supplements, such as methylB12 (methylcobalamin) or methylfolate.
Read more about COMT and supplement interactions here.
| Gene | RS ID | Effect Allele | Your Genotype | Notes About Effect Allele |
|---|---|---|---|---|
| COMT | rs4680 | A | -- | GG = higher activity; AG=Intermediate activity AA = lower activity |
| COMT | rs4633 | T | -- | CC = higher activity ; TT = lower COMT activity |
| COMT | rs6267 | T | -- | Minor decrease in COMT |
| COMT | rs165599 | A | -- | Minor decrease in COMT |
| COMT | rs165774 | A | -- | lower COMT activity; more likely to have irrational beliefs if subjected to maltreatment in childhood |
Cyanocobalamin is the common form of vitamin B12 found in many inexpensive vitamin brands. However, the ‘cyano’ in cyanocobalamin refers to a cyanide compound. Your body can detoxify this small amount of cyanide fairly easily, but many people choose to avoid the cyanide and take the active forms of B12, which include methylcobalamin (methylB12), adenosylcobalamin, or hydroxycobalamin.
Who might this work best for?
While getting enough vitamin B12 is important for everyone, it is probably more important for people with the MTR or MTRR variants, which directly use vitamin B12.
Taurine:
In a study of healthy women aged 33 -54, taurine supplementation lowered homocysteine levels. The average initial homocysteine was 8.5 umol/L. After 4 weeks of taking 3g of taurine per day, homocysteine decreased to an average of 7.6 umol/L.[ref]
Who might this work best for?
While taurine may help everyone a little bit, animal studies show that taurine is effective in lowering homocysteine levels when BHMT is impaired.[ref]
Related article: Taurine: Research on healthspan and supplements
N-Acetylcysteine (NAC):
NAC is a supplemental form of cysteine. A clinical trial involving 82 adults found that supplemental NAC (1.8g/day for 4 weeks) lowered plasma homocysteine levels.[ref]
Who might this work best for?
The clinical trial did not separate participants by genotype, so NAC may work for most people with high homocysteine. Animal studies suggest that NAC may be more important for NOX4 variants. In NOX4 knockout mice, cysteine helped protect the liver from acetaminophen injury.[ref]
Vitamin B6, pyroxidine:
Vitamin B6 is an essential cofactor in the transulfuration pathway with the CBS enzyme, which converts homocysteine to cysteine (and then to glutathione).
A clinical trial of folic acid plus vitamin B6 showed that the combination was effective in lowering homocysteine levels.[ref]
Who might this work best for?
If you have high levels of inflammation and oxidative stress, you may need more glutathione to act as an intracellular antioxidant. If you don’t get enough vitamin B6 in your diet, you may want to try a vitamin B6 supplement. The active form of B6 is called pyridoxal 5′-phosphate or P5P.
Related article: Vitamin B6, genetics, safety
Vitamin B2, riboflavin:
A clinical trial found that riboflavin (vitamin B2) was effective in reducing plasma homocysteine levels.[ref]
Who might this work for?
Riboflavin may be particularly helpful for people with the MTRR G allele.[ref] Additionally, studies show that riboflavin is particularly effective in people who are homozygous for MTHFR C677T (AA genotype).[ref]
Related article: MTR, MTRR and more about B12
The B vitamins together: B-complex for the win
Many studies show that a combination of folate, riboflavin, and/or vitamin B6 is very effective for lowering homocysteine.[ref][ref] A B-complex vitamin containing methylfolate and methylB12 may be the easiest way to go, especially if you don’t have the slow COMT variant.
Betaine or choline:
Methyl groups to remethylate homocysteine can also be supplied by betaine (also called TMG) or choline in the diet.[ref] Beets are a good source of betaine, and eggs are rich in choline.
Creatine:
The largest use of methyl groups is in the synthesis of creatine. Supplemental creatine can help to lower homocysteine levels in healthy people with good kidney function.[ref][ref][ref]
Related article: Creatine: Boosting muscles and cognitive function
Who might this work for?
Many of the studies on creatine for lowering homocysteine levels have been done in healthy people who were also lifting weights. If you don’t have any reason not to take it, such as kidney disease, creatine may be a good option for people with slow COMT variants who want to avoid methylfolate and methylB12 supplements. As a powder, creatine is inexpensive and easy to mix into any beverage (mild taste).
Antioxidants:
Low PON1 is associated with higher homocysteine. Antioxidant vitamins, C and E, or pomegranate juice, can increase PON1 levels.[ref] For people with the PON1 variant, you may want to consider how many antioxidant-rich foods you eat each day.
Keeping track:
If you are trying to lower homocysteine levels with supplements or dietary changes, I would suggest getting your homocysteine levels tested first to know your baseline. Then test again in 4-6 weeks to see if your interventions are making a difference. If you’re anything like me, it can sometimes be hard to remember why you’re taking a particular supplement. Write it down – perhaps on your printout of your homocysteine test results. Or start a spreadsheet with your lab results and your supplements or dietary changes. I also have a printable Supplements Tracker worksheet available for members.
Recap of your genes:
| Gene | RS ID | Effect Allele | Your Genotype | Notes About Effect Allele |
|---|---|---|---|---|
| MTHFR | rs1801133 | A | -- | MTHFR C677T, higher homocysteine levels, especially if folate is lacking |
| NOX4 | rs11018628 | C | -- | decreased homocysteine, decreased stroke risk |
| MTR | rs1805087 | G | -- | increased risk of cognitive impairment due to higher homocysteine |
| MTR | rs2275565 | T | -- | associated with higher homocysteine levels |
| MTRR | rs1801394 | G | -- | somewhat increased homocysteine levels, especially if riboflavin is low |
| CBS | rs5742905 | G | -- | risk of increased homocysteine, responsive to vitamin B6 |
| PON1 | rs662 | C | -- | higher homocysteine-thiolactone levels |
| PEMT | rs7946 | T | -- | TT: homocysteine increases with low folate diet |
| BHMT | rs3733890 | A | -- | reduced conversion of choline to betaine |
Related articles: