Key takeaways:
~ Mannose-binding lectin (MBL) is part of the innate immune system and help with pathogen recognition without relying on antibodies.
~ A deficiency in mannose-binding lectin can increase susceptibility to infections such as pneumonia, meningitis, and sinus infections.
~ Genetic variants in the MBL2 and MASP2 genes impact MBL levels and risk of infections by certain viruses.
Mannose-binding Lectin and the Immune System
The body has many ways to fight off different pathogens. You may be quite familiar with some pathogen-fighters, such as antibodies or T-cells, but the lectin pathway and mannose-binding lectin is a part of the immune system that is not as well known.
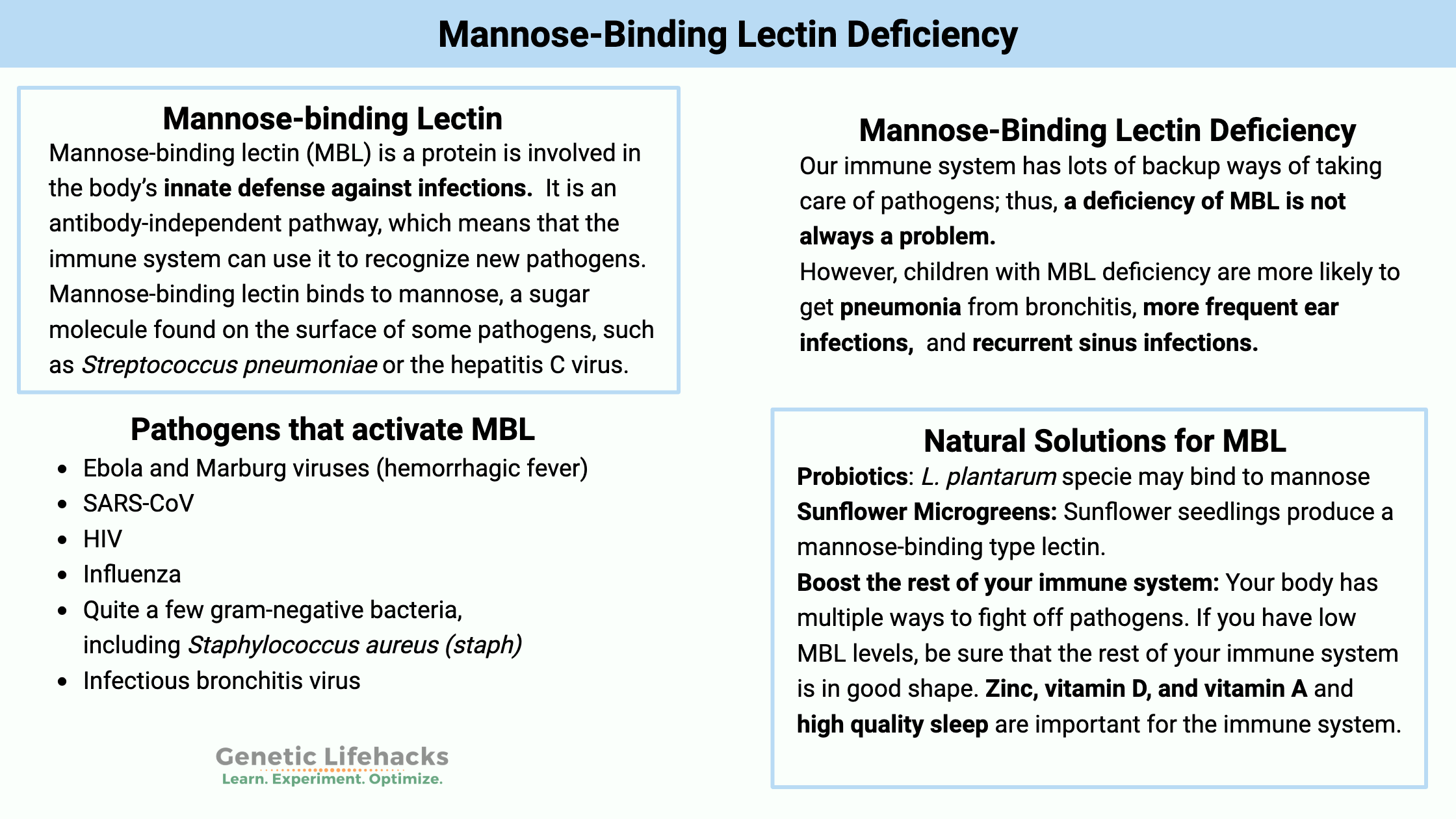
Mannose-binding lectin, also known as mannose-binding protein or mannan-binding protein, is involved in the body’s innate defense against infections. Find out how genetic variants in this system increase your susceptibility to specific pathogens.
Let’s start with some background information on lectins and the innate immune system, and clear up some possible misconceptions.
Lectin is a general term for a protein that binds to a carbohydrate.
Lectins are found in the body as part of the immune system and as cell receptors.
Much of what you read about lectins on alternative health blogs refers to plant lectins, especially those found in grains, legumes, and nightshades. There are lots of articles on why you should or should not eat lectins, but that isn’t what we are talking about here… Instead, mannose-binding lectin is a type of lectin produced in your body and used as part of the immune response.
A quick explanation of “alternative activation of the complement system”:
The lectin pathway is part of our immune system in the body. It is an antibody-independent pathway, which means that the immune system can use it to recognize new pathogens, not just those for which the body has already developed antibodies.
This part of the immune response is initiated by a pathogen membrane (such as the cell membrane of a bacterium or the envelope of a virus) that contains the carbohydrate mannose.
Mannose-binding lectin, abbreviated as MBL, binds to mannose, a sugar molecule found on the surface of some pathogens, such as Streptococcus pneumoniae or the hepatitis C virus.
To activate the immune system, though, you need a combination of mannose-binding lectin (MBL) plus two more proteins that bind to it.
The MASP1 (Mannose-binding lectin-associated serine protease 1) and MASP2 proteins join together with MBL, signaling that the viral or bacterial cell they are bound to is a pathogen that needs to be removed.
This combination of MBL, MASP1, and MASP2 activates the part of the immune system called the complement system. The complement system initiates a series of reactions that attack the pathogen’s cell membrane to kill the pathogen.
Which pathogens can activate mannose-binding lectin?
There are various bacteria, viruses, and microorganisms that can activate the complement system through MBL. These pathogens include:[ref][ref]
- Ebola and Marburg viruses (hemorrhagic fever)
- SARS-CoV
- HIV
- Influenza
- Quite a few gram-negative bacteria, including Staphylococcus aureus (staph)
- Infectious bronchitis virus
- SARS-CoV-2[ref]
Mannose-binding lectin and apoptosis:
In addition to its role in pathogen recognition, mannose-binding lectin is also essential in apoptosis, or how the body gets rid of cellular debris.[ref]
Symptoms of Mannose Binding Lectin Deficiency:
Genetically, people either produce less effective mannose-binding lectin (MBL) or have lower levels of MBL, depending on the variant.[ref]
Resiliency:
Our immune system has lots of backup ways of taking care of pathogens; thus, a deficiency of MBL is not always a problem. Often someone will not know that they have it.
However, low mannose-binding lectin can come into play in certain circumstances.
Studies show:
- MBL deficiency can be a problem for someone with a compromised immune system.[ref]
- Children with MBL deficiency have more frequent ear infections and/or upper respiratory infections.[ref] Not all studies show this, but most studies indicate a statistically higher rate of respiratory infections in kids.[ref]
- Children with genetically lower mannose-binding lectin are more likely to get pneumonia from bronchitis when compared to normal MBL. Children with normal MBL levels were more likely to have symptoms such as fever and bronchitis symptoms, but less likely to get pneumonia.[ref]
- There can be an increased risk of abscesses, meningitis, and sepsis with low MBL levels.[ref]
- Decreased MBL is linked to increased odds of recurrent sinus infections.[ref]
- In people with the flu (H1N1), low MBL levels are linked to milder symptoms.[ref]
- Many studies have looked at the role of MBL in HIV infections, as mannose-binding lectin can be important for preventing AIDS. MBL can bind to the surface of HIV, so it is being studied to determine if increased MBL affects the rate of progression of HIV.
Pros and cons: tradeoffs of higher MBL
It isn’t as cut-and-dry as MBL deficiency being ‘bad’. There are pros and cons associated with the amount of MBL in the body. Often a higher immune response is great for pathogens, but there are tradeoffs with inflammatory processes in the body.
- A 2014 mouse study found that MBL is involved in traumatic brain injuries; mice with MBL deficits had fewer sensorimotor deficits than mice with normal MBL.[ref]
- Other studies show that mannose-binding lectin may be involved in inflammation in blood cells, promoting inflammation there.[ref]
- Higher levels of mannose-binding lectin are linked with a greater risk of diabetic kidney disease (variants that cause low levels of MBL are protective against kidney disease in diabetes).[ref]
How common is mannose-biding lectin deficiency?
MBL deficiency is not all that rare. Some studies estimate that about 5% of people have undetectable levels of mannose-binding lectin, while another 30% have very low levels. It varies a bit by ancestry group.[ref]
Autoimmune diseases and MBL deficiency:
SLE (systemic lupus erythematosus) and RA (rheumatoid arthritis) are more common in people with low mannose-binding lectin.
Genetic variants in the MBL gene are also linked to an increased relative risk of Sjögren’s syndrome.[ref]
Covid and mannose-binding lectin:
I mentioned above that MBL can bind to the SARS-CoV-2 virus and activate the immune response. Specifically, mannose-binding lectin can activate certain aspects of the immune response, such as natural killer cells. MBL also modulates T cells and keeps the immune response under control.[ref]
Non-genetic causes of decreased mannose-binding lectin:
Genetic variants are the most frequent reason for low MBL levels, but there are a few other causes:
- Altered hormones, such as thyroid hormone or pituitary hormones can also impact MBL levels, independent of genetic variants.
- Hypothyroidism is linked to lower levels of MBL, while hyperthyroidism is associated with higher MBL levels. [ref]
- Hypopituitary hormones are also linked to significantly lower levels of MBL.[ref]
Mannose-binding lectin Genotype Report:
Lifehacks: Natural solutions for mannose-binding lectin deficiency
If you have lower MBL levels due to genetics, you may wonder if it is possible to raise your mannose-binding lectin level.
Research studies show that in addition to genetics, there are multiple ways that you can influence mannose-binding lectin levels in order to optimize immune response:
Thyroid hormone:
Good thyroid function is important for MBL levels.[ref] If you are hypothyroid, talk with your doctor about ways to correct your thyroid levels. Read through the full article on thyroid hormones and your genes to understand the different factors affecting thyroid function.
Weight loss?
Sometimes, knowing what doesn’t work is also important. Weight loss had no impact in one study on the MBL level.[ref]
Probiotics:
Several studies have looked at different Lactobacillus species, which can bind to mannose-binding lectins to prevent HIV and herpes simplex virus.[ref] Certain L. plantarum species seem to bind to mannose, but it appears that more studies need to be done on the subject.
Related Articles and Topics:
References:
Areeshi, Mohammed Y., et al. “A Meta-Analysis of MBL2 Polymorphisms and Tuberculosis Risk.” Scientific Reports, vol. 6, Nov. 2016, p. 35728. PubMed, https://doi.org/10.1038/srep35728.
Brown, Elizabeth E., et al. “MBL2 and Hepatitis C Virus Infection among Injection Drug Users.” BMC Infectious Diseases, vol. 8, May 2008, p. 57. PubMed Central, https://doi.org/10.1186/1471-2334-8-57.
Chen, Mengshi, et al. “Impact of Passive Smoking, Cooking with Solid Fuel Exposure, and MBL/MASP-2 Gene Polymorphism upon Susceptibility to Tuberculosis.” International Journal of Infectious Diseases: IJID: Official Publication of the International Society for Infectious Diseases, vol. 29, Dec. 2014, pp. 1–6. PubMed, https://doi.org/10.1016/j.ijid.2014.08.010.
Chong, Yong Pil, et al. “Association of Mannose-Binding Lectin 2 Gene Polymorphisms with Persistent Staphylococcus Aureus Bacteremia.” PLoS ONE, vol. 9, no. 3, Mar. 2014, p. e89139. PubMed Central, https://doi.org/10.1371/journal.pone.0089139.
Complement Deficiencies | Immune Deficiency Foundation. https://primaryimmune.org/about-primary-immunodeficiencies/specific-disease-types/complement-deficiencies/. Accessed 13 May 2022.
Høyem, P. H., et al. “The Effect of Weight Loss on Serum Mannose-Binding Lectin Levels.” Clinical and Developmental Immunology, vol. 2012, Nov. 2012, p. e354894. www.hindawi.com, https://doi.org/10.1155/2012/354894.
Https://Limo.Libis.Be/Primo-Explore/Fulldisplay?Docid=LIRIAS1759532&context=L&vid=Lirias&search_scope=Lirias&tab=default_tab&lang=en_US&fromSitemap=1. https://limo.libis.be/primo-explore/fulldisplay?docid=LIRIAS1759532&context=L&vid=Lirias&search_scope=Lirias&tab=default_tab&lang=en_US&fromSitemap=1. Accessed 13 May 2022.
Kjærup, Rikke M., et al. “Adjuvant Effects of Mannose-Binding Lectin Ligands on the Immune Response to Infectious Bronchitis Vaccine in Chickens with High or Low Serum Mannose-Binding Lectin Concentrations.” Immunobiology, vol. 219, no. 4, Apr. 2014, pp. 263–74. PubMed Central, https://doi.org/10.1016/j.imbio.2013.10.013.
Levy, Emily R., et al. “Evaluation of Mannose Binding Lectin Gene Variants in Pediatric Influenza Virus-Related Critical Illness.” Frontiers in Immunology, vol. 10, 2019, p. 1005. PubMed, https://doi.org/10.3389/fimmu.2019.01005.
Lin, Yong, et al. “Impact of Mannose-Binding Lectin 2 Polymorphism on the Risk of Hepatocellular Carcinoma: A Case-Control Study in Chinese Han Population.” Journal of Epidemiology, vol. 25, no. 5, May 2015, pp. 387–91. PubMed Central, https://doi.org/10.2188/jea.JE20140194.
Liu, Lei, and Bo Ning. “The Role of MBL2 Gene Polymorphism in Sepsis Incidence.” International Journal of Clinical and Experimental Pathology, vol. 8, no. 11, 2015, pp. 15123–27.
Longhi, Luca, et al. “Mannose-Binding Lectin Is Expressed after Clinical and Experimental Traumatic Brain Injury and Its Deletion Is Protective.” Critical Care Medicine, vol. 42, no. 8, Aug. 2014, pp. 1910–18. PubMed, https://doi.org/10.1097/CCM.0000000000000399.
Michelow, Ian C., et al. “High-Dose Mannose-Binding Lectin Therapy for Ebola Virus Infection.” The Journal of Infectious Diseases, vol. 203, no. 2, Jan. 2011, pp. 175–79. PubMed Central, https://doi.org/10.1093/infdis/jiq025.
Ogden, C. A., et al. “C1q and Mannose Binding Lectin Engagement of Cell Surface Calreticulin and CD91 Initiates Macropinocytosis and Uptake of Apoptotic Cells.” The Journal of Experimental Medicine, vol. 194, no. 6, Sept. 2001, pp. 781–95. PubMed, https://doi.org/10.1084/jem.194.6.781.
Orsini, Franca, et al. “Mannose-Binding Lectin Drives Platelet Inflammatory Phenotype and Vascular Damage After Cerebral Ischemia in Mice via IL (Interleukin)-1α.” Arteriosclerosis, Thrombosis, and Vascular Biology, vol. 38, no. 11, Nov. 2018, pp. 2678–90. ahajournals.org (Atypon), https://doi.org/10.1161/ATVBAHA.118.311058.
Rashidi, Elahe, et al. “Mannose-Binding Lectin Deficiency in Patients with a History of Recurrent Infections.” Iranian Journal of Allergy, Asthma and Immunology, Jan. 2016, pp. 69–74. ijaai.tums.ac.ir, https://ijaai.tums.ac.ir/index.php/ijaai/article/view/628.
Regente, Mariana, et al. “A Sunflower Lectin with Antifungal Properties and Putative Medical Mycology Applications.” Current Microbiology, vol. 69, no. 1, July 2014, pp. 88–95. PubMed, https://doi.org/10.1007/s00284-014-0558-z.
Riis, Anne Lene Dalkjær, et al. “Thyroid Hormone Increases Mannan-Binding Lectin Levels.” European Journal of Endocrinology, vol. 153, no. 5, Nov. 2005, pp. 643–49. eje.bioscientifica.com, https://doi.org/10.1530/eje.1.02013.
Rs1800450 – SNPedia. https://snpedia.com/index.php/Rs1800450. Accessed 13 May 2022.
Rs72550870 – SNPedia. https://snpedia.com/index.php/Rs72550870. Accessed 13 May 2022.
Ruskamp, Jopje M., et al. “Mannose-Binding Lectin and Upper Respiratory Tract Infections in Children and Adolescents: A Review.” Archives of Otolaryngology–Head & Neck Surgery, vol. 132, no. 5, May 2006, pp. 482–86. Silverchair, https://doi.org/10.1001/archotol.132.5.482.
Tong, Xiang, et al. “Association between the Mannose-Binding Lectin (MBL)-2 Gene Variants and Serum MBL with Pulmonary Tuberculosis: An Update Meta-Analysis and Systematic Review.” Microbial Pathogenesis, vol. 132, July 2019, pp. 374–80. PubMed, https://doi.org/10.1016/j.micpath.2019.04.023.
Tu, Xinyi, et al. “Functional Polymorphisms of the CCL2 and MBL Genes Cumulatively Increase Susceptibility to Severe Acute Respiratory Syndrome Coronavirus Infection.” The Journal of Infection, vol. 71, no. 1, July 2015, pp. 101–09. PubMed, https://doi.org/10.1016/j.jinf.2015.03.006.
UpToDate. https://www.uptodate.com/contents/mannose-binding-lectin? Accessed 13 May 2022.
Zhang, Nana, et al. “Association of Levels of Mannose-Binding Lectin and the MBL2 Gene with Type 2 Diabetes and Diabetic Nephropathy.” PLoS ONE, vol. 8, no. 12, Dec. 2013, p. e83059. PubMed Central, https://doi.org/10.1371/journal.pone.0083059.
Ziółkowska, Natasza E., and Alexander Wlodawer. “Structural Studies of Algal Lectins with Anti-HIV Activity.” Acta Biochimica Polonica, vol. 53, no. 4, 2006, pp. 617–26.