Key takeaways:
~NAD+ is a critical coenzyme, utilized in every living cell and essential for numerous cellular functions.
~ NAD+ levels decrease with aging, and this is thought to be one of the key reasons for many age-related declines.
~ Nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN) are available as supplements and increase NAD+ levels.
~ Genetic variants impact the body’s production of NAD+ and related genes.
This article digs into the science of how NR and NMN work, the research that has been done on NR and NMN, and then explains the connections with aging of sirtuins, PARPs, and CD38 or CD157.
Members will see their genotype report below and the solutions in the Lifehacks section. Consider joining today.What is NAD+?
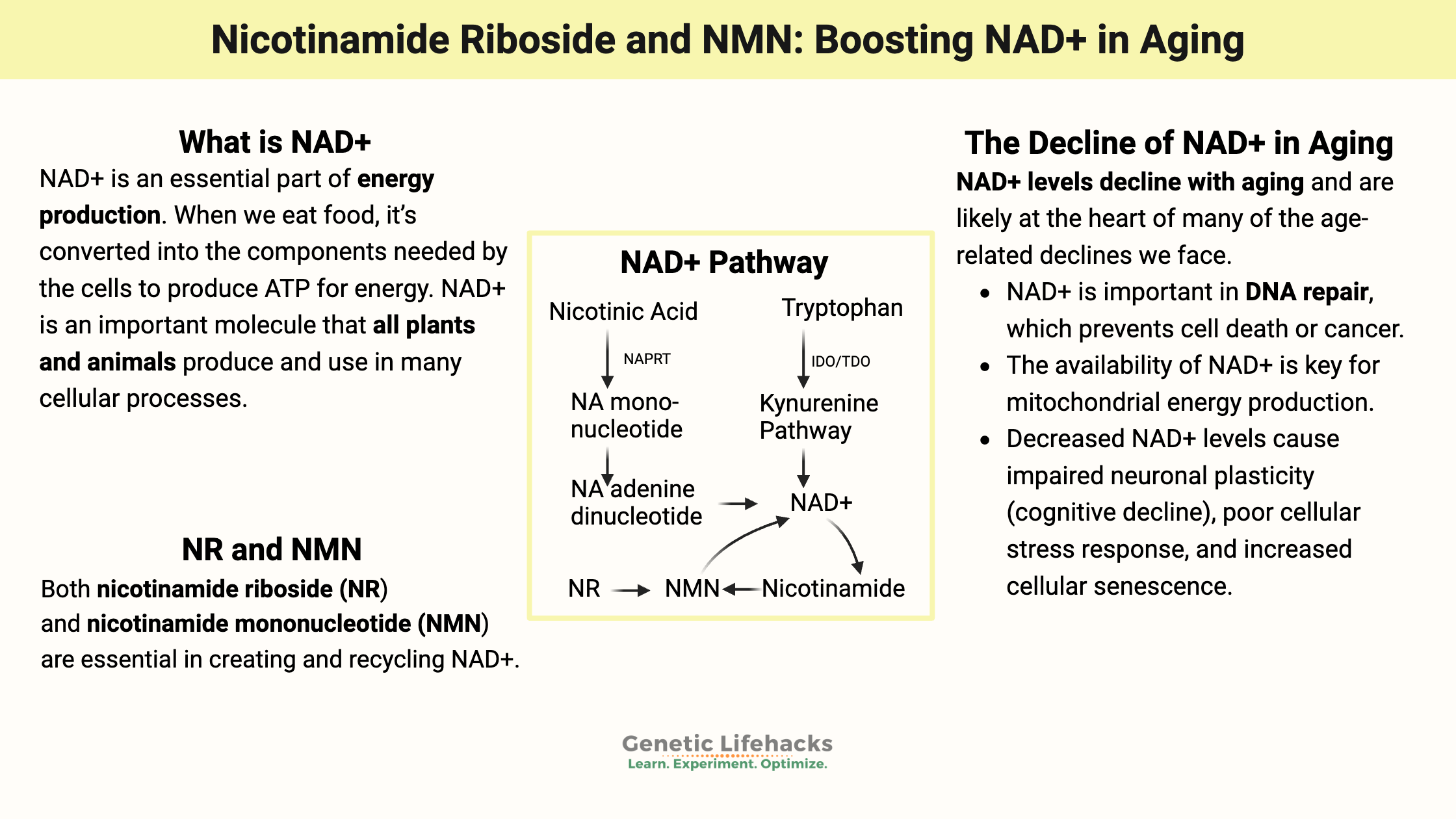
NAD+ (nicotinamide adenine dinucleotide) is an important molecule that all plants and animals produce and use in cellular processes. It is a niacin derivative indispensable for multiple cellular functions, earning its reputation as a molecule essential for life.
First, let’s look at NAD+ in ATP production and then touch on the many other roles.
NAD+ in cellular energy production:
A quick overview of the basics of cellular energy production…
In cellular metabolism, NAD+ is an essential part of energy production. When we eat food, it’s converted into the components needed by the cells to produce ATP for energy. For example, carbohydrates break down into glucose, which can then be directly used in cells to produce ATP, the molecule your body uses for energy storage.
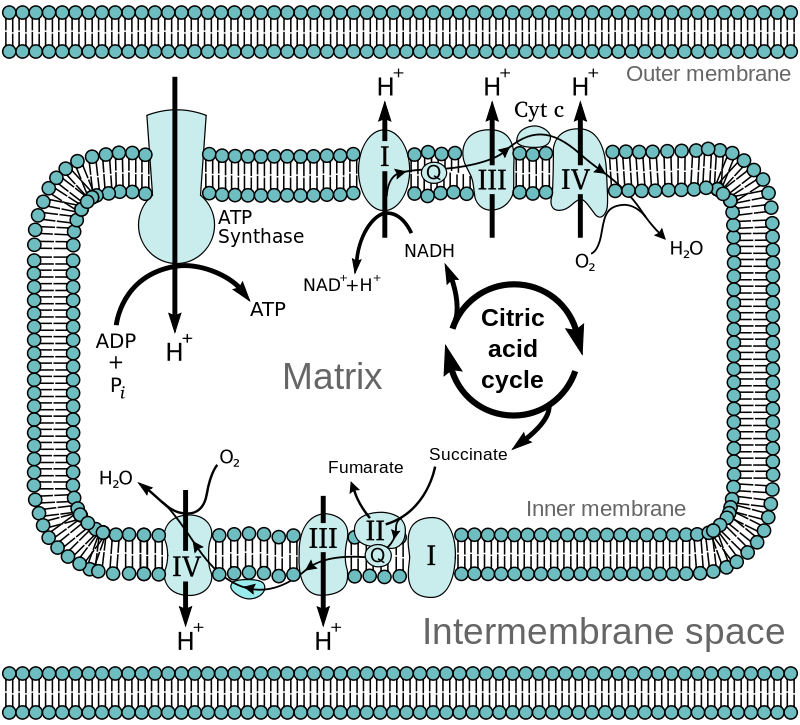
During cellular energy production, the majority of ATP production comes from processes in the mitochondria (the powerhouse of the cell). This is where NAD+ is essential.
Two ways ATP is made in mitochondria, and both involve NAD+:
- Within the Krebs cycle (a.k.a. citric acid cycle), electrons shuttle between NAD+ and NADH.
- Within the inner membrane of the mitochondria, the electron transport chain uses NAD+ for the transfer of electrons. This is your body’s main way of producing energy when enough oxygen is present.
Here’s what this looks like:
Other essential roles of NAD+:
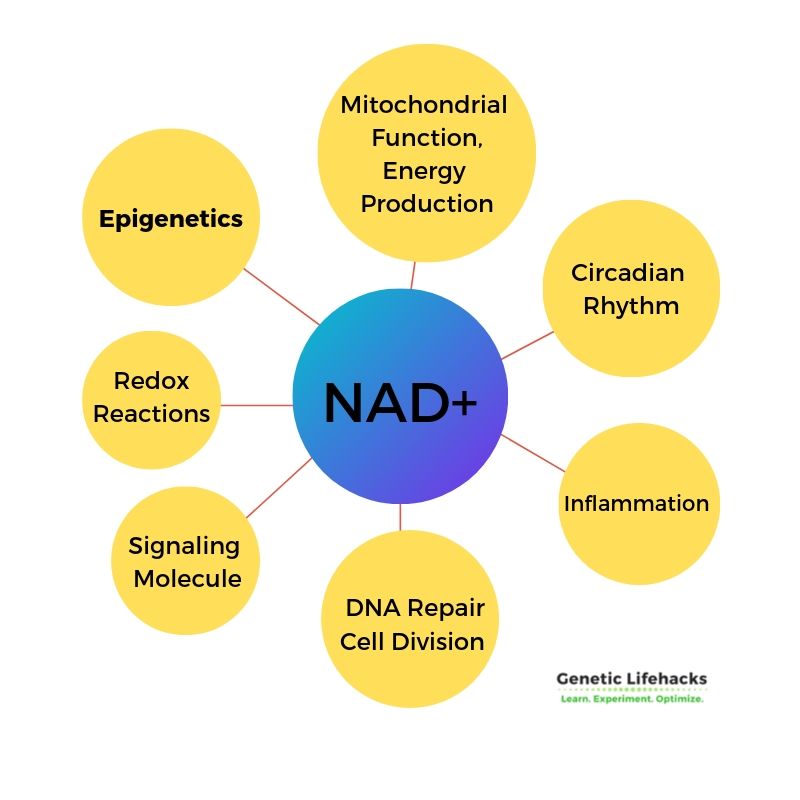
Beyond energy production, NAD+ is also used in numerous other reactions in the body.
1) ADP-ribose transfer reactions consume NAD+. ADP-ribose transfer reactions include:
- The repair of DNA
- The maintenance of telomeres
- PARP and CD38/CD157 reactions
2) CD38/CD157: Immune system and NAD+
Two very similar and related enzymes, CD157 and CD38, are responsible for using up a lot of NAD+ in the body.
- CD157 acts both as an enzyme and as a cellular receptor on immune cells. It is important in immune system reactions, including in the brain. Research ties variants in CD157 to Parkinson’s disease and REM sleep disorder. Studies also find that CD157 variants impact autism risk related to the regulation of brain development.[ref]
- Interestingly, researchers have found in animal studies that CD157 (also called BST1) is important in oxytocin levels in the brain. The study was specifically looking at how low oxytocin relate to autism spectrum behaviors. Giving NR to mice that lack CD157/BST1 corrected the behavioral deficits in the animals.[ref] A recent study in children with autism found that nicotinamide riboside levels (along with 7 other metabolites) are lower in autism spectrum disorder compared to healthy controls.[ref]
- CD38 is a very similar molecule to CD157 and acts in much the same way in different cell types. Both molecules use up NAD+ by acting as enzymes that catalyze the degradation of NAD+ to use the metabolites as messenger molecules.
- CD157 and CD38 also play an important role in the immune system and are found as surface receptors on immune cells. Both are also involved in the immune response and neuroinflammation in the brain.[ref]
3) Sirtuins:
Sirtuins are a family of proteins (SIRT1 through SIRT7) essential for turning on and off the translation of genes within a cell. For instance, SIRT1 is linked to nutrient sensing and insulin resistance, and its enhancement can help resist obesity-related problems. Other sirtuins, like SIRT6, play roles in metabolic regulation and mitochondrial function. All sirtuins depend on NAD+ for their activity, linking NAD+ levels to energy availability in organisms.
4) Cell signaling processes:
Additionally, NAD+ is involved in cell signaling processes both within and outside of cells.
The decline of NAD+ in aging:
As we age, there are a lot of physiological changes that take place. We all know the signs of aging: hearing loss and hair loss; your muscle mass declines and wrinkles increase; weight tends to rise, along with blood glucose levels. Eventually, everything seems to go downhill… And a lack of NAD+ is part of the problem.
NAD+ levels decline with aging and are likely at the heart of many of the age-related declines we face.[ref]
What does NAD+ do that is important in aging?
- NAD+ is important in DNA repair, which prevents cell death or cancer.[ref]
- Mitochondrial energy production decreases as we age is another big part of why everything goes downhill.
- The availability of NAD+ is key for mitochondrial energy production.
- Decreased NAD+ levels cause impaired neuronal plasticity (cognitive decline), poor cellular stress response, and increased cellular senescence.
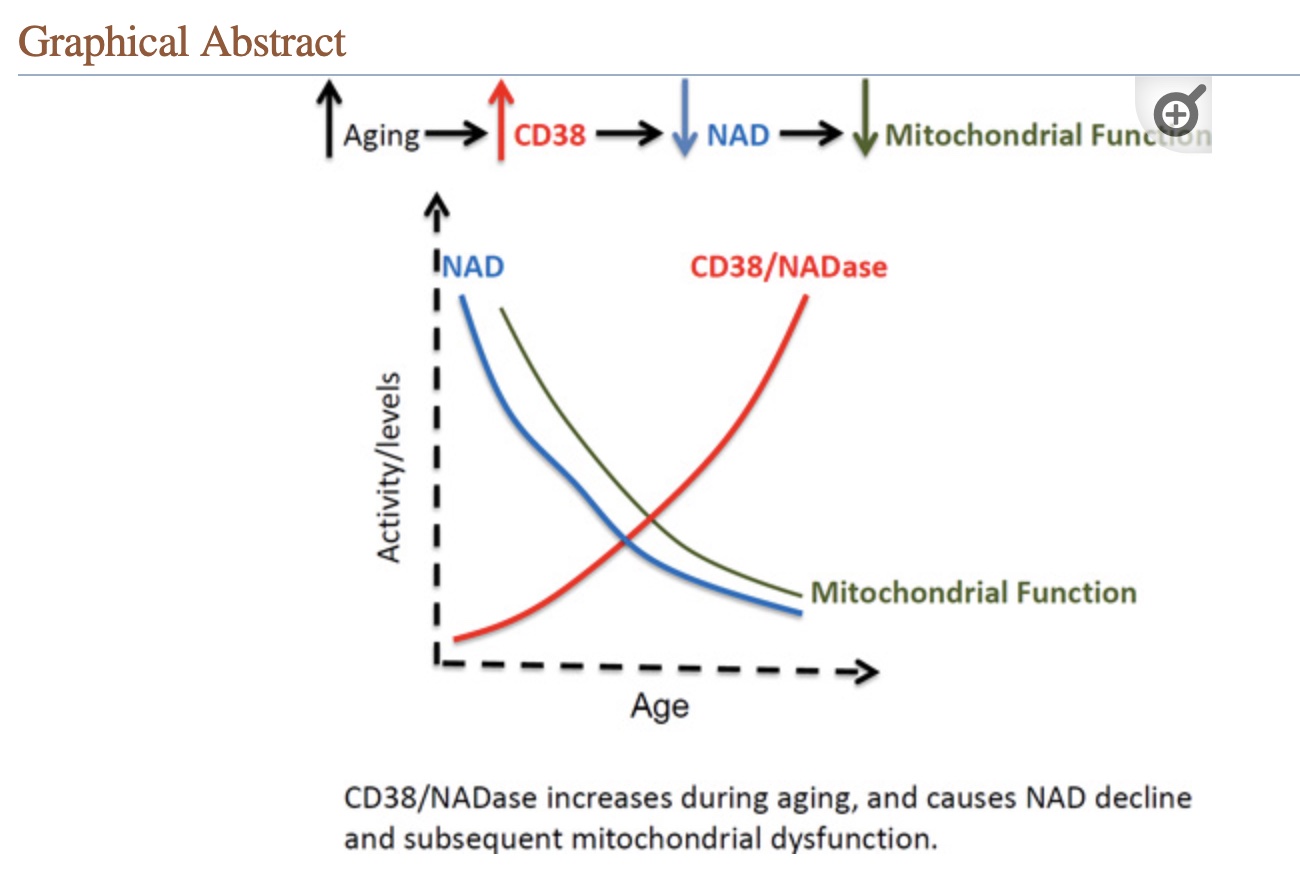
CD38 and Aging:
Research shows that CD38 is a key part of the decline of NAD+ in aging. Knocking out the CD38 gene can prevent NAD+ declines in aging.
In aging, increased inflammation causes increased CD38, which uses up more NAD+, causing a parallel decline in NAD+ levels. Increased cellular senescence in aging also causes increased CD38 (and decreased NAD+).[ref]
Should we all just block CD38 to prevent the decline in NAD+ levels? Well, I don’t think so… CD38 does a lot, including regulating calcium ions and neurotransmitter release. It’s also important in the activation of T cells. So while it isn’t as simple as just shutting down all CD38 activity, I’ll include some ways to decrease CD38 activity a little bit in the Lifehacks section. (More on CD38 and CD157 in the Genotype report section)
Nicotinamide Riboside, NAD+ pathway, and Covid: Why Covid hits older adults hard
NAD+ levels interact with the immune response in Covid patients in a couple of ways:
- When someone is ill with COVID-19, the PARP genes are over-expressed, and NAD levels decrease.
- Researchers find that in COVID-19, PARPs are increased, the NAD+ salvage pathway is activated, and NAD biosynthesis (e.g., niacin creation) is decreased. One study concludes, “These data suggest that the antiviral activities of noncanonical PARP isozyme activities are limited by the availability of NAD and that nutritional and pharmacological interventions to enhance NAD levels may boost innate immunity to coronaviruses.”[ref]
- CD38 levels are broadly upregulated in Covid patients, which further depletes NAD+ levels.[ref]
- A 2023 study showed that the gene expression of multiple NAD-consuming enzymes was increased (PARPs, CD38) while SIRT1 expression was downregulated. This leads to a significant decrease in NAD+ levels (which are already decreased in aging). Animal models clearly showed that boosting NAD+ could improve recovery from Covid.[ref]
Sirtuins and Aging:
I mentioned above that sirtuins rely on NAD+, which is important in gene expression. Let me explain this further…
Sirtuins are a family of genes (SIRT1 through SIRT7) involved in regulating gene expression. The sirtuins cause the DNA in the cell nucleus to be either accessible or inaccessible for a gene to be transcribed.
The ability for the regulation of genes to be transcribed into proteins is fundamental to cell function. Every cell contains the same DNA in the nucleus. The differences between a liver cell and a muscle cell are due to the regulation of which genes are transcribed. Thus, disrupting the sirtuins can lead to mucked-up cell function and the symptoms of aging.
Lifespan extension studies:
In the initial studies on the sirtuin genes in yeast, adding additional copies of the gene increased lifespan by 30%.[ref] This discovery led to research showing how important sirtuins are in human healthspan.
The SIRTs all have specific functions:
- SIRT1 encodes the sirtuin 1 protein. It involves sensing nutrient availability and is thus linked to problems with insulin resistance. Studies show that animals with insulin resistance have decreased SIRT1 levels. When researchers increase SIRT1 in animals, they are resistant to the problems of obesity and insulin resistance that a high-fat diet induces in them.[ref][ref][ref]
- SIRT2 codes for the sirtuin 2 protein, which arranges the chromosomes for cell division in mitosis.
- SIRT3, 4, and 5, found in the mitochondria, are important for oxidative stress and fat metabolism.[ref]
- SIRT6 is important in gene expression for metabolic regulation, telomere maintenance, and mitochondrial respiration. Reducing Sirt6 in the liver causes animals to develop fatty liver disease, and knocking out Sirt6 altogether causes animals to die within a few weeks due to severely accelerated aging.[ref]
Sirtuins use NAD+ to complete their cellular activity, and through that, the NAD+ levels may be a sensor for how much energy is available in an organism.[ref] (More on the SIRTs in the Genotype section below)
PARPs and NAD+ in aging:
Another enzyme group that utilizes NAD+ in their reactions is PARPs, which stands for poly(ADP-ribose) polymerase.
PARPs are another family of proteins that are important in DNA repair and genomic stability. They detect broken DNA and signal for it to be repaired. Additionally, when DNA isn’t able to be repaired, cell death is initiated. Again, these are vital cellular functions, especially in aging.[ref]
PARP1 uses up a lot of NAD+ in the process, causing a decrease in ATP production for the cell. When a cell hasn’t replicated the DNA properly, the DNA damage signaling response is enacted.[ref] Cell death is necessary in the right context, but excessive cell death, especially in the brain, is not good.
Excessive DNA breakage can lead to a lot of PARP activation, thus depleting NAD+.
What causes DNA breakage? UV light, reactive oxygen species (oxidative stress), lipid peroxidation, and a lot of different environmental toxicants. DNA damage occurs all the time in the normal course of cell replication, but in aging, oxidative stress causes an increase in PPAR1 and a subsequent decrease in NAD+. PARP1 can initiate cellular repair for single-strand DNA breaks. This is important for longevity. Inhibiting PARP is a way to mitigate the decreased NAD+ and ATP levels and decrease cell death. It doesn’t fix the cause (DNA breakage), but it puts a band-aid on the downstream effects of PARP activation.
Atherosclerosis and congestive heart failure are two diseases in which PARP inhibitors might be used. The inflammation within the vascular cells causes PARP1 activation and the subsequent decrease in NAD+ and cellular energy. Inhibiting PARP then slows the inflammatory response and preserves the ATP and NAD+ in the heart cells.[ref][ref]
Creating NAD+ in the body:
Precursors of NAD+ include different forms of niacin (vitamin B3) and tryptophan.
The different forms of niacin, whether from food or supplements, are nicotinamide (also called niacinamide) and nicotinic acid (called niacin). The nicotinic acid form (niacin) is the one that can cause flushing when taken as a supplement.
What happens if you don’t get enough niacin from food? A lack of niacin causes a disease state known as pellagra. In the early 1900s, when people in the US South were dependent on corn (no niacin) for most of their calories, there was an epidemic of pellagra. Symptoms of pellagra include dementia, diarrhea, and a skin rash.
Tryptophan is an essential amino acid. Your body can also convert tryptophan into niacin through the kynurenine pathway. However, this pathway to form niacin isn’t usually enough to meet the body’s needs, and thus, you also need to get niacin from food.
In addition to using niacin from tryptophan, another way cells create more NAD+ is by converting nicotinic acid. The first step in converting nicotinic acid to NA mononucleotide (NAM) uses the NAPRT enzyme coded for by the NAPRT gene.[ref][ref]
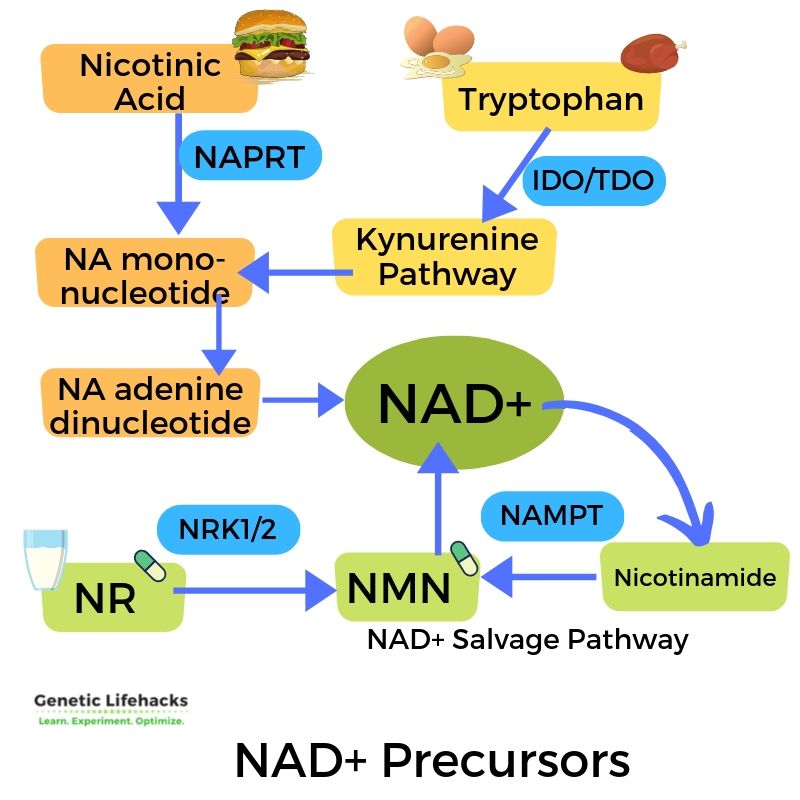
NR (Nicotinamide Riboside) and NMN (Nicotinamide Mononucleotide):
Both nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN) are essential in creating and recycling NAD+. Plus, both are available as supplements. Let’s look at how they come into play in the NAD+ pathway – and why supplemental NR or NMN may be important in aging.
NMN, synthesized from nicotinamide (niacinamide) and PRPP (5’-phosphoribosyl-pyrophosphate), uses the enzyme NAMPT.[ref] (More on NAMPT in the Genotype section below.)
Nicotinamide riboside (NR) is another precursor of NAD+ and an intermediate in the NAD salvage pathway. It can be found at low levels in foods, particularly in milk, and it is available as a supplement.
NAD+ doesn’t have to be synthesized continually from the precursors — it can be recycled through the “NAD Salvage Pathways“. Reusing the components of NAD+, specifically nicotinamide, is your body’s main way of having enough NAD+ available in all cells. This salvage pathway uses the supplemental NR and NMN in the body.
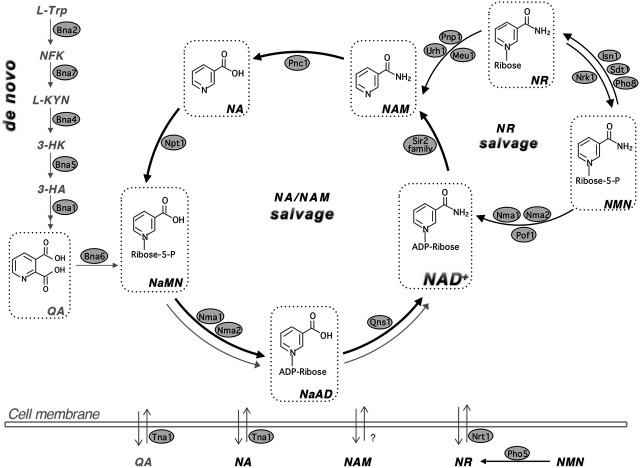
from PMC8171187, CC 4.0
This salvage pathway is where supplemental NR and NMN come into play — boosting NAD+ levels. This salvage process hinges on a key enzyme, NAMPT.
Research studies on supplemental NR and NMN:
Enough background science – let’s get into the research on supplemental nicotinamide riboside and nicotinamide mononucleotide and how they can affect the diseases of aging.
Animal studies on NR and NMN:
Below are some of the animal studies on NAD+ precursors (NR or NMN):
- Reverses Alzheimer’s in animals: In a mouse model of Alzheimer’s disease, NMN shows the restoration of mitochondrial function in the brain. The oxygen consumption deficits in the brain mitochondria, found in Alzheimer’s, showed a reversal. Again, this is a mouse study… but pretty cool.[ref]
- Restores Fertility: Several studies have shown that NMN or NAD+ precursors restore fertility at the end of an animal’s normal reproductive age. It seems to do this through rejuvenating egg quality.[ref][ref]
- Counteracts hemorrhagic shock: In a rodent model of hemorrhagic shock, those receiving NMN had less inflammation, better cellular metabolism, and increased survival in hemorrhagic shock.[ref]
- Decreases signs of aging: Nicotinamide riboside (NR) was fed to old mice for three months. The NR decreased several of the signs of aging in mice, such as altered fat mass, cholesterol levels, and liver enzymes. [ref]
- Reverses fatty liver disease: Quite a few studies show that NR can reverse fatty liver disease.[ref][ref][ref]
- Improves cognitive function: Another mouse study showed that NR could improve cognitive function in a mouse and reduce inflammatory markers in the brain.[ref]
- Protects against hearing loss: NR was shown to protect mice from age-related noise-induced hearing loss by increasing SIRT3 expression.[ref]
- Prevents retinal degeneration: NR helps to prevent retinal degeneration and inflammation in the retina. [ref]
- Boosts growth: In mice, NR increased lactation, nursing behavior, and transmission of micronutrients to the mouse babies. Those offspring grew up to have advantages “in physical performance, anti-anxiety, spatial memory, delayed onset of behavioral immobility, and promotion of adult hippocampal neurogenesis”.[ref]
- Improves mitochondrial function: A mouse study also found that NMN could dampen the DNA damage response and improve mitochondrial function.[ref]
- Increased lifespan: A small increase in lifespan (about 4%) has been shown in mice fed NR starting at old age.[ref]
- Restored SIRT1 levels: Middle-aged mice fed NMN showed increased Sirt1 levels, similar to younger mice.[ref]
Clinical trials (in humans) using NR and NMN:
Animal studies are great for showing the mechanism of action, but human clinical trials give us a better picture of what happens at different doses, ages, and health conditions.
1) Decreased Inflammation:
A study of ‘aged men’ looked at the effects of supplementing with 1,000 mg of NR per day for three weeks. The results showed an elevation of NAD+ in the muscles and a decrease in inflammatory cytokine levels.[ref]
2) Heart health:
A study that included 30 middle-aged and older men and women looked at the effect of NR vs placebo for six weeks. Oral NR supplementation (1,000mg/day ) raised NAD+ levels by 60% compared to placebo. NR lowered blood pressure and aortic stiffness (a little). Notably, participants who had stage one hypertension to begin with had a 10-point drop in systolic blood pressure.[ref]
A small clinical trial in patients with heart failure showed that oral nicotinamide riboside decreased proinflammatory cytokines. The researchers concluded that NR may improve mitochondrial respiration and attenuate inflammation in heart failure.[ref]
3) Brain health:
A study of NR supplementation (500 mg, 2x /day, 6 weeks) in older adults showed increased NAD+ levels in neuronal vesicles and decreased neuroinflammatory markers.[ref]
4) Skeletal muscle metabolism:
A recent study found: “NR supplementation of 1000 mg/d for 6 wk in healthy overweight or obese men and women increased skeletal muscle NAD+ metabolites, affected skeletal muscle acetylcarnitine metabolism, and induced minor changes in body composition and sleeping metabolic rate.”[ref]
5) Fighting off infections:
A clinical trial found that people recovered more quickly from COVID-19 when given supplemental NR, N-acetyl-cysteine, and l-carnitine. The phase II and phase III clinical trials found that recovery was 3-4 days faster with the supplement.[ref]
6) Part of the Alzheimer’s stack:
An exciting phase II clinical trial using a combination of natural supplements showed improved cognitive function in people diagnosed with Alzheimer’s disease. The combination included 1 g of nicotinamide riboside, 2.5 g NAC, 3.7 g l-carnitine, and 12g of l-serine.[ref]
7) Insulin secretion:
A small study using 250 mg of NMN daily, before breakfast, found that it helped to attenuate insulin secretion after eating sugar.[ref]
8) Premature aging prevention:
A study in people with Werner syndrome, a genetic disease that causes premature aging, showed that nicotinamide riboside could prevent some of the premature aging, including helping with kidney function.[ref]
Safety and side effects of NR and NMN supplements:
The first question is always – is it safe? So far, studies show that NR and NMN are safe, for most people. Keep in mind that these studies last months to years, so the long-term effects of taking NR or NMN are based just on animal studies. Talk with your doctor if you have questions about supplements.
- A study looked at the safety of nicotinamide riboside (TruNiagen brand) taken by healthy men and women for 8 weeks, in doses ranging from 100 to 1000 mg. All doses increased NAD+ metabolites within two weeks, which was dose-dependent. Most importantly, there were no differences in adverse events between the NR groups and the placebo group. [ref]
- A 6-month randomized, double-blind, placebo-controlled clinical trial with NR plus pterostilbene found that it was safe and well tolerated. While it didn’t cure NAFLD (fatty liver) in six months, there was a significant improvement in liver enzymes, ALT and GGT, in the NR group compared to placebo. [ref]
- Another trial found that 2,000 mg/day of NR in obese, sedentary men aged 40 – 70 was safe and well tolerated in a 12-week study. [ref] Note that 2,000 mg/day is a higher dose than most supplements, which range from 100-300 mg.
- In a safety trial for people with Parkinson’s disease, the patients took 1,500 mg of nicotinamide riboside daily. “NR therapy was well tolerated with no moderate or severe adverse events, and no significant difference in mild adverse events.”[ref]
Does NR or NMN promote atherosclerotic plaque?
A 2024 study made headlines with evidence that niacin increases atherosclerotic plaque for some people with specific genetic variants. The study was focusing more on dietary niacin than other sources, however, a 2025 study in mice also showed that higher doses of NR increased atherosclerotic plaque through the same pathway.[ref]
While more studies are needed, I would highly suggest reading through the article on Niacin and Atherosclerosis and checking your genes.
Does NR or NMN mess up methylation?
Some health gurus on the internet have theorized that taking NR or NMN will mess up your methylation cycle. While it makes sense in theory, this study in adults supplementing with nicotinamide riboside noted that NR did not mess up methylation.[ref]
Does NR or NMN actually boost NAD+?
A clinical trial examined the effects of NR on healthy volunteers for 9 days. The study participants took 250 mg for the first two days and then were titrated up to 1000 mg. On day 9, NAD+ levels had increased by 100%. No side effects were reported for the NR supplement. Interestingly, most of the individual response curves were similar in percentage increase, but a couple of participants had a much bigger response.[ref]
Another clinical trial looked at NMN to see if it increased NAD+ levels. The results showed that 300, 600, and 900mg doses significantly increased NAD+ levels. It was safe and well tolerated, and subjective health improved statistically in the group taking NMN compared to the placebo group.[ref]
Circadian rhythm depends on NAD+
Circadian rhythm is the 24-hour biological rhythm that controls many cellular functions. It is estimated that about 40% of cell functions fluctuate over the course of a day – based on circadian clock gene expression.
The core molecular circadian clock is driven by the rising and falling levels of four genes: CLOCK and BMAL1 rise and then are suppressed as PER and CRY accumulate. The CLOCK gene expression is controlled by a sirtuin (SIRT1), which, in turn, is dependent on NAD+ levels.[ref]
I know – this article is getting a bit deep in the science, but stick with me. Let me connect a few dots…
NAD+ levels are needed for the sirtuins to work. The sirtuin family of proteins controls whether a portion of the DNA is available to be transcribed or not. Like a light switch turning on or off.
SIRT1, important for the core circadian gene, CLOCK, to function correctly, rises and falls cyclically over 24 hours. SIRT1 relies on NAD+ availability.[ref]
The disruption of the core clock genes is causally linked to chronic diseases of aging, such as diabetes, heart disease, obesity, metabolic syndrome, and Alzheimer’s disease.[ref]
Thus, one mechanism by which low NAD+ levels impact us as we age is through altered CLOCK gene expression.
SIRT6 has also been shown to control the liver’s clock – separately from SIRT1. It leads to the control of lipid metabolism in the liver, which ties circadian rhythm, NAD+, and metabolic health together.[ref]
NAD+ Genotype Report:
Lifehacks:
NAD+ levels decline with aging, and reversing this decline has been shown in numerous studies (mostly animal studies) to help with overall health in aging.
Niacin, NR, or NMN from food:
Niacin can be formed in the body from a pathway that starts with tryptophan. Abundant in most protein-containing foods, tryptophan is an essential amino acid that can either be converted into kynurenine (and eventually niacin) or serotonin.
Some studies indicate that 20mg/day of niacin can meet our need for NAD+ biosynthesis. The US RDA is 16mg/day.[ref]
NMN is found in trace amounts in foods: Broccoli and cabbage contain up to 1mg/ 100 gm of NMN, and avocados and tomatoes also have trace amounts. While food can be a minor source of NMN, it is mainly synthesized in the body.
Tryptophan, the precursor amino acid for niacin, can also eventually be converted to NAD+. But, it takes 60 times the amount of tryptophan compared to niacin. Tryptophan can help to prevent pellagra (niacin deficiency disease), but it isn’t the main source for most people today.[ref]
Related article: Tryptophan and the kynurenine pathway genes
Supplements to boost NAD+:
There are multiple ways to boost NAD+ levels with supplements, from directly supplying NR or NMN to decreasing the breakdown of NAD in cells. Additionally, you need to take into account your age in considering whether you even need to worry about NAD+ levels. Please talk with your doctor if you have any questions on whether a supplement is right for you.
Related Articles and Topics:
HPA Axis Dysfunction: Understanding Cortisol and Genetic Interactions
NQO1 Gene: Metabolism of quinones, benzene, and more
Your genes code for the enzymes that break down the toxic substances we encounter each day. This ability to clear out potential carcinogens is important for preventing cancer. The NQO1 gene codes for an enzyme that breaks down quinones, benzene, and some chemotherapy drugs.
Metformin
A decades-old diabetes drug now holds promise for increasing healthspan. Research shows that metformin may reduce the risk of some of the diseases of aging, thus increasing the number of years someone is healthy.
HPA Axis Dysfunction: Cortisol and Stress
Cortisol is a hormone produced by the adrenal glands in times of stress, and it also plays many roles in your normal bodily functions. It is a multi-purpose hormone that needs to be in the right amount (not too high, not too low) and at the right time. Your genes play a big role in how likely you are to have problems with cortisol.
Intermittent Fasting: Benefits from changing Gene Expression
The intermittent fasting concept has gained traction in health circles. Learn more about the importance of when you eat and its effects on gene expression.
References:
Airhart, Sophia E., et al. “An Open-Label, Non-Randomized Study of the Pharmacokinetics of the Nutritional Supplement Nicotinamide Riboside (NR) and Its Effects on Blood NAD+ Levels in Healthy Volunteers.” PLoS ONE, vol. 12, no. 12, Dec. 2017, p. e0186459. PubMed Central, https://doi.org/10.1371/journal.pone.0186459.
Altay, Ozlem, et al. “Combined Metabolic Activators Accelerates Recovery in Mild-to-Moderate COVID-19.” Advanced Science (Weinheim, Baden-Wurttemberg, Germany), vol. 8, no. 17, Sept. 2021, p. e2101222. PubMed, https://doi.org/10.1002/advs.202101222.
Amano, Hisayuki, et al. “Telomere Dysfunction Induces Sirtuin Repression That Drives Telomere-Dependent Disease.” Cell Metabolism, vol. 29, no. 6, June 2019, pp. 1274-1290.e9. PubMed, https://doi.org/10.1016/j.cmet.2019.03.001.
Bertoldo, Michael J., et al. “NAD+ Repletion Rescues Female Fertility during Reproductive Aging.” Cell Reports, vol. 30, no. 6, Feb. 2020, pp. 1670-1681.e7. www.cell.com, https://doi.org/10.1016/j.celrep.2020.01.058.
Brown, Kevin D., et al. “Activation of SIRT3 by the NAD⁺ Precursor Nicotinamide Riboside Protects from Noise-Induced Hearing Loss.” Cell Metabolism, vol. 20, no. 6, Dec. 2014, pp. 1059–68. PubMed, https://doi.org/10.1016/j.cmet.2014.11.003.
Cantó, Carles, et al. “NAD+ Metabolism and the Control of Energy Homeostasis – a Balancing Act between Mitochondria and the Nucleus.” Cell Metabolism, vol. 22, no. 1, July 2015, pp. 31–53. PubMed Central, https://doi.org/10.1016/j.cmet.2015.05.023.
Clement, James, et al. “The Plasma NAD+ Metabolome Is Dysregulated in ‘Normal’ Aging.” Rejuvenation Research, vol. 22, no. 2, Apr. 2019, pp. 121–30. liebertpub.com (Atypon), https://doi.org/10.1089/rej.2018.2077.
Conze, Dietrich, et al. “Safety and Metabolism of Long-Term Administration of NIAGEN (Nicotinamide Riboside Chloride) in a Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Healthy Overweight Adults.” Scientific Reports, vol. 9, no. 1, July 2019, p. 9772. PubMed, https://doi.org/10.1038/s41598-019-46120-z.
Dollerup, Ole L., et al. “A Randomized Placebo-Controlled Clinical Trial of Nicotinamide Riboside in Obese Men: Safety, Insulin-Sensitivity, and Lipid-Mobilizing Effects.” The American Journal of Clinical Nutrition, vol. 108, no. 2, Aug. 2018, pp. 343–53. PubMed, https://doi.org/10.1093/ajcn/nqy132.
Duarte-Pereira, Sara, et al. “NAMPT and NAPRT1: Novel Polymorphisms and Distribution of Variants between Normal Tissues and Tumor Samples.” Scientific Reports, vol. 4, Sept. 2014, p. 6311. PubMed Central, https://doi.org/10.1038/srep06311.
Ear, Po Hien, et al. “Maternal Nicotinamide Riboside Enhances Postpartum Weight Loss, Juvenile Offspring Development, and Neurogenesis of Adult Offspring.” Cell Reports, vol. 26, no. 4, Jan. 2019, pp. 969-983.e4. ScienceDirect, https://doi.org/10.1016/j.celrep.2019.01.007.
Elhassan, Yasir S., et al. “Nicotinamide Riboside Augments the Aged Human Skeletal Muscle NAD+ Metabolome and Induces Transcriptomic and Anti-Inflammatory Signatures.” Cell Reports, vol. 28, no. 7, Aug. 2019, pp. 1717-1728.e6. PubMed, https://doi.org/10.1016/j.celrep.2019.07.043.
Gariani, Karim, et al. “Eliciting the Mitochondrial Unfolded Protein Response by Nicotinamide Adenine Dinucleotide Repletion Reverses Fatty Liver Disease in Mice.” Hepatology (Baltimore, Md.), vol. 63, no. 4, Apr. 2016, pp. 1190–204. PubMed Central, https://doi.org/10.1002/hep.28245.
Gerasimenko, Maria, et al. “Nicotinamide Riboside Supplementation Corrects Deficits in Oxytocin, Sociability and Anxiety of CD157 Mutants in a Mouse Model of Autism Spectrum Disorder.” Scientific Reports, vol. 10, 2020. www.ncbi.nlm.nih.gov, https://doi.org/10.1038/s41598-019-57236-7.
Guan, Yi, et al. “Nicotinamide Mononucleotide, an NAD+ Precursor, Rescues Age-Associated Susceptibility to AKI in a Sirtuin 1–Dependent Manner.” Journal of the American Society of Nephrology, vol. 28, no. 8, Aug. 2017, pp. 2337–52. jasn.asnjournals.org, https://doi.org/10.1681/ASN.2016040385.
Han, Xue, et al. “Nicotinamide Riboside Exerts Protective Effect against Aging-Induced NAFLD-like Hepatic Dysfunction in Mice.” PeerJ, vol. 7, 2019, p. e7568. PubMed, https://doi.org/10.7717/peerj.7568.
Henning, Robert J., et al. “Poly(ADP-Ribose) Polymerase (PARP) and PARP Inhibitors: Mechanisms of Action and Role in Cardiovascular Disorders.” Cardiovascular Toxicology, vol. 18, no. 6, Dec. 2018, pp. 493–506. PubMed, https://doi.org/10.1007/s12012-018-9462-2.
Hong, Guangliang, et al. “Administration of Nicotinamide Riboside Prevents Oxidative Stress and Organ Injury in Sepsis.” Free Radical Biology & Medicine, vol. 123, Aug. 2018, pp. 125–37. PubMed, https://doi.org/10.1016/j.freeradbiomed.2018.05.073.
Kane, Alice E., and David A. Sinclair. “Sirtuins and NAD+ in the Development and Treatment of Metabolic and Cardiovascular Diseases.” Circulation Research, vol. 123, no. 7, Sept. 2018, pp. 868–85. PubMed Central, https://doi.org/10.1161/CIRCRESAHA.118.312498.
Kang, Dae-Wook, et al. “Distinct Fecal and Plasma Metabolites in Children with Autism Spectrum Disorders and Their Modulation after Microbiota Transfer Therapy.” MSphere, vol. 5, no. 5, Oct. 2020, pp. e00314-20. PubMed, https://doi.org/10.1128/mSphere.00314-20.
Lee, Hee Jae, and Soo Jin Yang. “Supplementation with Nicotinamide Riboside Reduces Brain Inflammation and Improves Cognitive Function in Diabetic Mice.” International Journal of Molecular Sciences, vol. 20, no. 17, Aug. 2019, p. E4196. PubMed, https://doi.org/10.3390/ijms20174196.
Long, Aaron N., et al. “Effect of Nicotinamide Mononucleotide on Brain Mitochondrial Respiratory Deficits in an Alzheimer’s Disease-Relevant Murine Model.” BMC Neurology, vol. 15, Mar. 2015, p. 19. PubMed Central, https://doi.org/10.1186/s12883-015-0272-x.
Lopatina, Olga L., et al. “CD157 and Brain Immune System in (Patho)Physiological Conditions: Focus on Brain Plasticity.” Frontiers in Immunology, vol. 11, 2020. www.ncbi.nlm.nih.gov, https://doi.org/10.3389/fimmu.2020.585294.
Martens, Christopher R., et al. “Chronic Nicotinamide Riboside Supplementation Is Well-Tolerated and Elevates NAD+ in Healthy Middle-Aged and Older Adults.” Nature Communications, vol. 9, no. 1, Mar. 2018, p. 1286. www.nature.com, https://doi.org/10.1038/s41467-018-03421-7.
Masri, Selma, et al. “Partitioning Circadian Transcription by SIRT6 Leads to Segregated Control of Cellular Metabolism.” Cell, vol. 158, no. 3, July 2014, pp. 659–72. PubMed Central, https://doi.org/10.1016/j.cell.2014.06.050.
Murata, Michael M., et al. “NAD+ Consumption by PARP1 in Response to DNA Damage Triggers Metabolic Shift Critical for Damaged Cell Survival.” Molecular Biology of the Cell, vol. 30, no. 20, Sept. 2019, pp. 2584–97. PubMed, https://doi.org/10.1091/mbc.E18-10-0650.
Nevoral, Jan, et al. “Epigenetic and Non-Epigenetic Mode of SIRT1 Action during Oocyte Meiosis Progression.” Journal of Animal Science and Biotechnology, vol. 10, Aug. 2019, p. 67. PubMed Central, https://doi.org/10.1186/s40104-019-0372-3.
Pfluger, Paul T., et al. “Sirt1 Protects against High-Fat Diet-Induced Metabolic Damage.” Proceedings of the National Academy of Sciences, vol. 105, no. 28, July 2008, pp. 9793–98. www.pnas.org, https://doi.org/10.1073/pnas.0802917105.
Remie, Carlijn M. E., et al. “Nicotinamide Riboside Supplementation Alters Body Composition and Skeletal Muscle Acetylcarnitine Concentrations in Healthy Obese Humans.” The American Journal of Clinical Nutrition, vol. 112, no. 2, Aug. 2020, pp. 413–26. PubMed, https://doi.org/10.1093/ajcn/nqaa072.
Salic, Kanita, et al. “Combined Treatment with L-Carnitine and Nicotinamide Riboside Improves Hepatic Metabolism and Attenuates Obesity and Liver Steatosis.” International Journal of Molecular Sciences, vol. 20, no. 18, Sept. 2019, p. E4359. PubMed, https://doi.org/10.3390/ijms20184359.
Sims, Carrie A., et al. “Nicotinamide Mononucleotide Preserves Mitochondrial Function and Increases Survival in Hemorrhagic Shock.” JCI Insight, vol. 3, no. 17, p. e120182. PubMed Central, https://doi.org/10.1172/jci.insight.120182. Accessed 15 Dec. 2021.
Sun, Cheng, et al. “SIRT1 Improves Insulin Sensitivity under Insulin-Resistant Conditions by Repressing PTP1B.” Cell Metabolism, vol. 6, no. 4, Oct. 2007, pp. 307–19. www.cell.com, https://doi.org/10.1016/j.cmet.2007.08.014.
Yaku, Keisuke, et al. “BST1 Regulates Nicotinamide Riboside Metabolism via Its Glycohydrolase and Base-Exchange Activities.” Nature Communications, vol. 12, 2021. www.ncbi.nlm.nih.gov, https://doi.org/10.1038/s41467-021-27080-3.
Zhang, Hongbo, et al. “NAD+ Repletion Improves Mitochondrial and Stem Cell Function and Enhances Life Span in Mice.” Science, vol. 352, no. 6292, June 2016, pp. 1436–43. science.org (Atypon), https://doi.org/10.1126/science.aaf2693.
Zhang, Xian, et al. “Systemic Treatment With Nicotinamide Riboside Is Protective in a Mouse Model of Light-Induced Retinal Degeneration.” Investigative Ophthalmology & Visual Science, vol. 61, no. 10, Aug. 2020. www.ncbi.nlm.nih.gov, https://doi.org/10.1167/iovs.61.10.47.
Zhou, Bo, et al. “Boosting NAD Level Suppresses Inflammatory Activation of PBMCs in Heart Failure.” The Journal of Clinical Investigation, vol. 130, no. 11, Nov. 2020, pp. 6054–63. PubMed, https://doi.org/10.1172/JCI138538.
https://academic.oup.com/edrv/article/31/2/194/2354747#59027139. Accessed 15 Dec. 2021.
https://www.genecards.org/cgi-bin/carddisp.pl?gene=NAPRT. Accessed 15 Dec. 2021.