Key takeaways:
~ ADHD affects around 5-7% of the population.
~ Brain imaging studies show physiological differences in the way certain regions of the brain work.
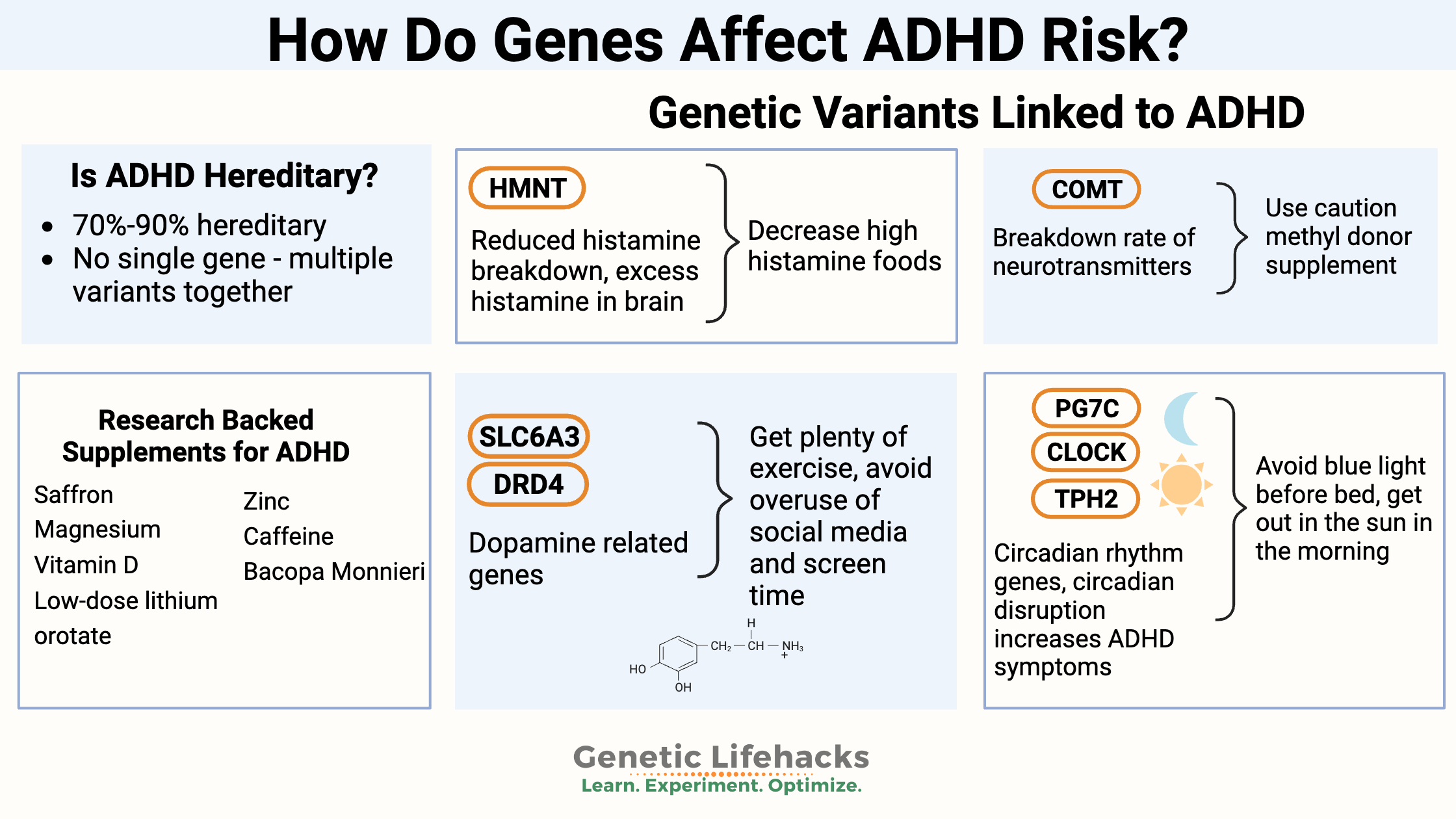
~ Many genetic variants come together to increase susceptibility to ADHD. You can check your 23 and Me or AncestryDNA data below for these genetic markers.
~Two pathways in ADHD genetic susceptibility include circadian rhythm genes and neurotransmitter (dopamine, norepinephrine, histamine) genes.
~ Environmental factors also play a role, including toxicant exposure.
How do genes affect ADHD risk?
ADHD (attention deficit hyperactivity disorder) is a condition that usually starts in childhood. It affects around 5% of kids around the world.
Symptoms include inattention, impulsivity, and hyperactivity. Studies show that two-thirds of kids with ADHD will still deal with these issues into adulthood, which can lead to other problems like dropping out of school, getting rejected by peers, injuries, getting in trouble with the law, not doing well in jobs, divorce, and even higher rates of suicide.[ref]
Heredity and ADHD:
Is ADHD hereditary? Twin studies show that the heritability of ADHD is 70 – 90% for inattentiveness and hyperactivity.[ref][ref] Heritability is a term that includes genetic variants along with epigenetics and in-utero exposure.
There is no single “ADHD” gene. Instead, researchers have discovered multiple genetic variants that contribute in small ways to the condition.
Genes related to dopamine, circadian rhythm, neuronal formation, serotonin transporters, tryptophan, and the breakdown of neurotransmitters have all been identified as playing a small role in ADHD. The small changes from multiple variants add up to form the risk for ADHD. It’s called a polygenic risk, meaning from multiple gene variants.[ref]
Rare mutations linked to ADHD:
Rare gene mutations have also been investigated to see if they cause ADHD, and it is likely that for a small percentage of people, a rare genetic condition causes it.
ADHD is found at a much higher rate in people with genetic chromosomal abnormalities, including Klinefelter Syndrome (XXY chromosomes), Williams Syndrome (partial deletion in chromosome 7), Turner Syndrome (missing X chromosome), or Fragile X syndrome.[ref]
Additionally, rare mutations in genes identified as ADHD candidate genes are found in higher numbers in people with ADHD.[ref]
In other words: Rare mutations may have a large impact on ADHD for an individual, but it is hard to determine this statistically when looking at a large population group.
Just a little more distracted than average:
Some researchers contend that ADHD is part of the continuum of normal behavior — one end of the spectrum. Their conclusion: “The data suggest that ADHD is best viewed as the quantitative extreme of genetic and environmental factors operating dimensionally throughout the distribution of ADHD symptoms, indicating that the same etiologic factors are involved in the full range of symptoms of inattention, hyperactivity and impulsivity.”[ref]
How is the ADHD brain different?
Brain imaging studies show physiological differences in the brains of people with ADHD.
PET scans and SPECT imaging showed that ADHD patients on psychostimulants had increased striatal dopamine transporter density. However, subjects not on stimulant medications had lower dopamine transporter density.[ref]
Another large study found that certain regions of the brain had differences in the cortical surface area in children with ADHD. Specifically, changes were found in the frontal cortex region.[ref] The frontal cortex is responsible for decision-making, reasoning, social appropriateness, and complex cognitive behaviors.
Biochemical pathways involved in ADHD:
The latest ADHD genetics research shows that two major pathways are likely involved:
- Dopamine modifications in the striatal neurons
- Altered circadian rhythm
These two pathways are clearly seen in genetic markers related to ADHD (details in the genotype section below).
1) Dopamine and Neurotransmitters
The dopamine pathways have been extensively researched in ADHD, which is what methylphenidate (Ritalin) acts on.
The dopamine reuptake transporter (DAT) is found in the striata and is the place where methylphenidate works. Scientists say that more DAT transporters in the striata, caused by genes or the environment, may be the cause of ADHD. However, other results don’t agree, showing the inconsistency of ADHD’s molecular physiology. [ref]
Dopamine is made from tyrosine utilizing the enzyme tyrosine hydroxylase (TH). Animal studies show that TH is reduced in the striatum of ADHD rats. Treadmill exercise increased TH and decreased ADHD.[ref]
2) Circadian rhythm alterations:
While dopamine is integrally related to ADHD symptoms, such as focus and working memory, ADHD patients often also have circadian rhythm abnormalities, including sleep problems.
Circadian rhythm is the 24-hour built-in body clock. In addition to sleep/wake cycles, your circadian rhythm controls the rise and fall of hormones such as cortisol and neurotransmitters such as dopamine. Researchers estimate that about 40% of the body’s molecular processes are controlled by the circadian clock.
A recent study looked at gene expression of core circadian clock genes along with the 24-hour profiles of cortisol and melatonin production in people with ADHD. The results showed significant differences in sleep patterns, cortisol rhythm, and the expression of core circadian clock genes (PER2 and BMAL1) in the ADHD group.[ref]
An earlier study found that adults with ADHD had altered BMAL1 and PER2 expression (core circadian rhythm genes).[ref]
Circadian rhythm interacts with dopamine as well. In a cell study using fibroblasts from people with ADHD, researchers found that dopamine significantly altered PER3 levels (core circadian clock gene). This change was not found in cell samples from people without ADHD.[ref] Additionally, the circadian clock regulates the production of enzymes that break down neurotransmitters, such as MAOA.[ref]
Related article: MAOA and the Warrior Gene
Environmental factors in ADHD
ADHD is not explained solely by genetics. When it comes to the neurocognitive changes in the ADHD brain, environmental factors that combine with genetic susceptibility are most likely at work.
Cortisol and Inflammatory markers:
The idea that neural inflammation is at the root of ADHD has been examined in many ways. Conflicting results have been shown on inflammatory biomarkers, with some studies showing slightly increased inflammation and others showing decreased inflammatory markers in children and adults with ADHD.
A review of 19 studies showed that, on average, cortisol levels are lower in youths with ADHD than is typical. Cortisol rises and falls over the course of a day, and the research showed that cortisol levels were lower throughout the day, as well as in cumulative levels.
Additionally, inflammatory markers such as TNF-alpha and IL-1B were also statistically a little lower in kids with ADHD when looking at the combined study data.[ref]
Cortisol response in kids with ADHD is different, though, than in kids without ADHD. A study looked at the response to parental expressed emotions on the kids. The expressed parental emotions caused a greater rise and then fall in cortisol in the kids with ADHD than in kids without ADHD.[ref]
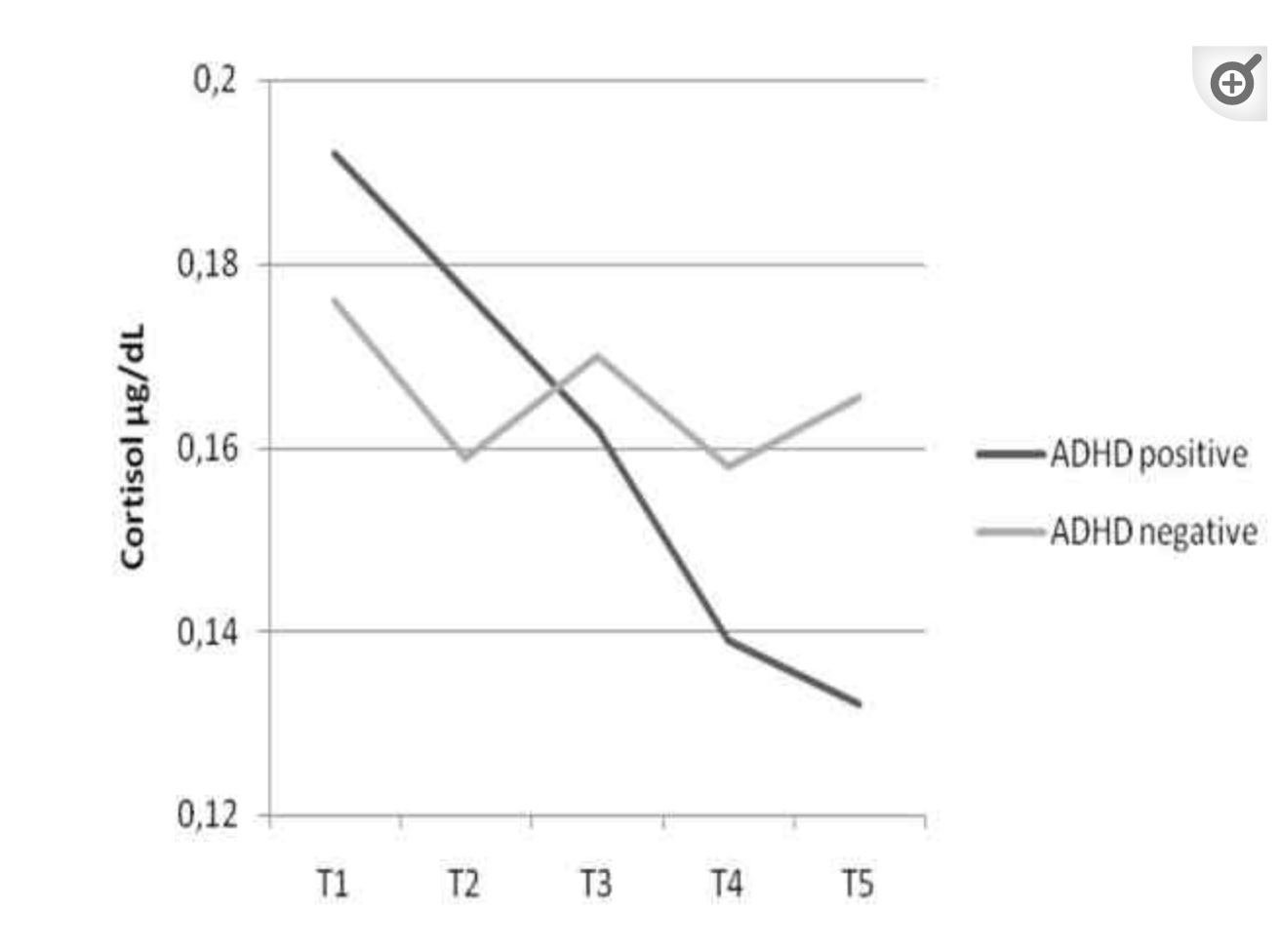
While most study results don’t show that elevated inflammatory cytokines are a hallmark of ADHD, neuroinflammation may still be a possible cause of ADHD symptoms for individuals. Targeting neuroinflammation may be more important for kids with ADHD who also have altered pain perception and pain sensitivity.[ref]
Exposures to toxicants before birth:
Some researchers theorize that environmental disruptions in the fetal environment can impact the developing nervous system, increasing the risk of ADHD and other neurodevelopmental disorders.
Maternal Smoking:
A review of multiple studies found that maternal smoking increased ADHD. There was also a link between getting a serious bacterial or viral infection (e.g., requiring hospitalization) while pregnant and an increased risk of the child developing ADHD.[ref][ref][ref]
Perfluorooctanoic Acid (PFOAs):
PFOAs are man-made persistent chemicals used as stain repellants and food wrapping (to repel oil). Prenatal exposure to PFOA at a higher level was linked to a 3-fold increase in the relative risk of ADHD in children.[ref]
Exposures in childhood:
Phthalates:
Exposure to higher levels of phthalates has been linked to increased ADHD susceptibility in several studies.[ref] Phthalates are common chemicals found in artificial fragrances, adhesives, vinyls, lotions, nail polish, food packaging, and even in boxed mac and cheese.[ref][ref]
Insecticide:
Pyrethroid exposure in children is linked to ADHD. Pyrethroids are a class of chemicals used as a pesticide, mostly as a home insecticide as well as for mosquito control. The study found that higher urinary pyrethroid metabolite levels corresponded to increased ADHD, especially impulsivity in boys.[ref]
Lead:
Exposure to lead during early childhood is also linked in many studies to an increased risk of ADHD. While not all studies show this link, the majority of studies in a meta-analysis did show a link between lead exposure, even at low levels, and ADHD.[ref]
Nutrient deficiencies: Can a supplement help ADHD
It would be nice if research showed that a kid with ADHD just needed more of a vitamin or mineral… And a lot of time and money has gone into figuring out whether there is simply a missing element.
Over the past couple of decades, research has shown contradictory results for many different vitamins and minerals. Magnesium, for example, was shown to be a little lower, on average, in kids with ADHD than in kids without ADHD. However, clinical trials on supplemental magnesium don’t show that it has much of an effect — except in kids who are truly deficient.
The evidence seems a little stronger that children with ADHD are likely to have lower zinc and iron levels than the control group without ADHD. Again, supplementation studies don’t show that restoring mineral levels effectively mitigates symptoms.[ref][ref][ref]
I want to point out, though, that what holds true for a group of kids with ADHD may not be true for an individual. It is possible that magnesium, zinc, or iron could be key for an individual who is deficient in that mineral.
What happens when ADHD kids grow up?
Studies show that 60-70% of kids with ADHD still have problems with symptoms as adults. While some kids may ‘grow out of it’, around two-thirds will still deal with ADHD as an adult. This speaks to the need for lifestyle adaptations and natural options for managing ADHD as an adult.
But what happens as you head toward old age? In general, brain volume decreases with aging. However, studies show there is less brain shrinkage in people over 60 who have been diagnosed previously with ADHD. It is hard to know, though, whether ADHD itself is neuroprotective or if the medications for ADHD are having an effect on brain volume.[ref]
Problems that go along with ADHD:
Whether due to overlapping genetic susceptibility or other factors, research shows that people with ADHD are at an increased risk of other mental health disorders:[ref]
- Increased risk of substance misuse disorders
- 9-fold increased risk of problematic media use (teens)
- Eating disorders are increased in ADHD
- Increased risk of migraines
- 2 to 3-fold increased risk of epilepsy
High histamine and ADHD:
A large meta-analysis looked at the overlap between atopic disease and ADHD. Atopic diseases include atopic dermatitis (eczema), allergic rhinitis, and asthma. The analysis included data from 38 studies with over 100,000,000 participants. The results showed that atopic diseases were increased in kids with ADHD compared to kids without ADHD.[ref]
Why is this important – the overlap of eczema, sinus allergies, and asthma with ADHD? It could mean that the underlying pathways involved in ADHD are also involved in atopic diseases. Atopic diseases are connected to inflammation and Th1, Th2, and Th17 immune responses. This ties into excess IgE and histamine production.
Histamine acts as a neurotransmitter in the brain. Histamine levels rise in the morning hours, making us feel alert when we wake up. (diphenhydramine makes you sleepy because it blocks the histamine receptors in the brain…).
Animal studies show that the HNMT (histamine n-methyltransferase) enzyme is essential for breaking down histamine in the brain. Brain histamine acts on various functions, including appetite, stress response, sleep-wake cycles, and memory.[ref]
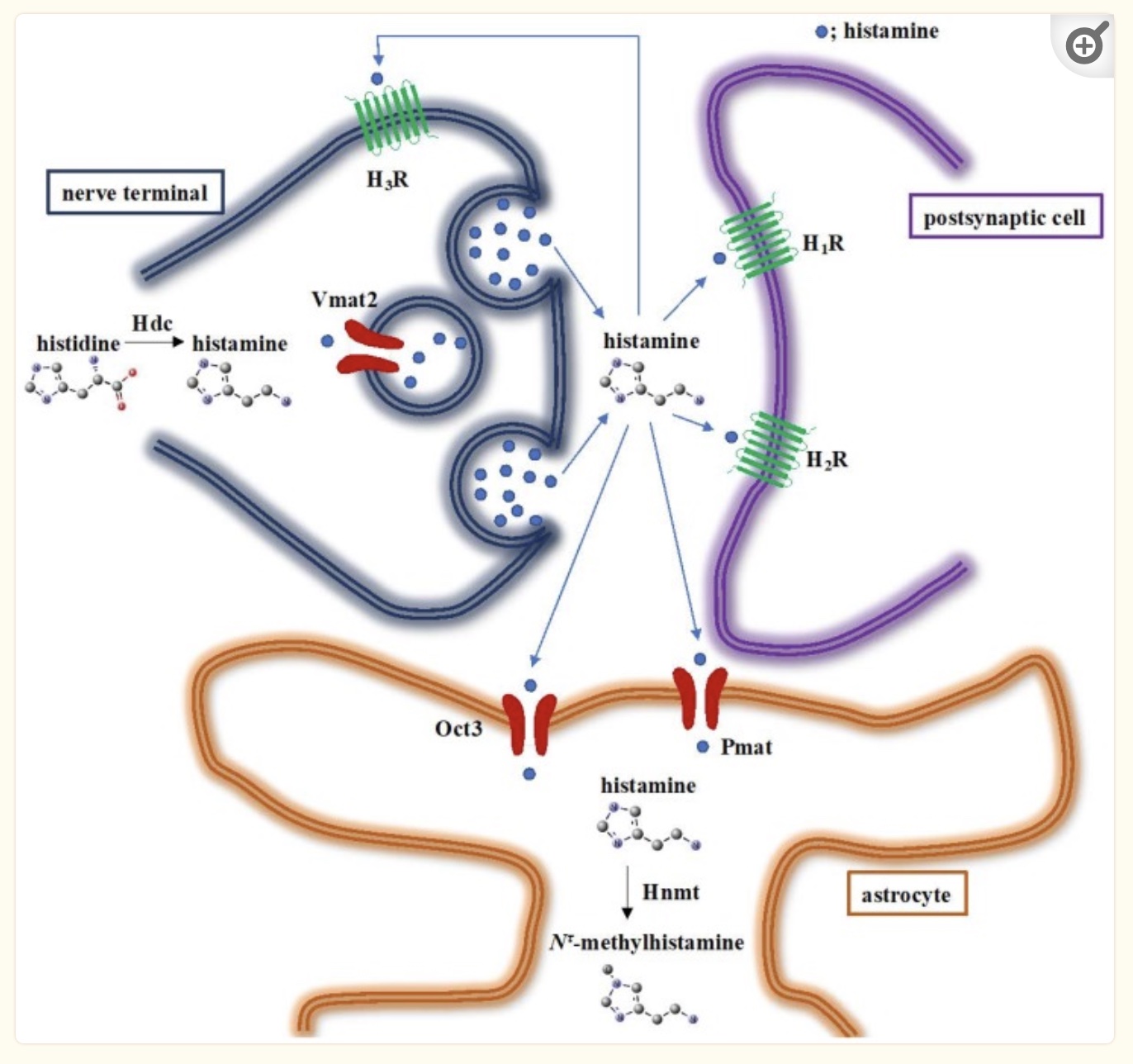
Interestingly, one of the genetic variants related to higher histamine levels in the brain (the HNMT gene) is linked to ADHD susceptibility.[ref] Another study showed that kids with a specific HNMT variant were sensitive to food coloring additives, including red and yellow dyes, relating ADHD to food additive reactions.[ref]
That which should not be spoken of…
I’m going to touch on the research on two more controversial aspects of ADHD research. Please click through to the referenced studies for more in-depth information.
Vaccinations:
A study involving over 4,000 kids examined the question of whether exposure to thimerosal-containing vaccinations (hepatitis B) increased the risk of ADHD. After adjusting for a bunch of variables (demographics, socioeconomics, health issues), researchers found that kids who were vaccinated with the thimerosal-containing hepatitis B vaccine had almost twice the risk of ADHD. The thimerosal-containing hep B vaccine was given between 1991 and 2001 to infants in the US.[ref]
Thimerosal is a mercury-based preservative widely used for decades in vaccines. However, starting in the early 2000s, thimerosal was removed from almost all childhood vaccines (the exception being the flu vaccine). The FDA states that thimerosal is safe in vaccines.[ref]
A study of Tdap-vaccination in pregnant women shows no statistical difference in ADHD rates of their children.[ref]
Acetaminophen usage in pregnancy:
Another interesting connection to rising ADHD levels may be prenatal exposure to acetaminophen. Essentially, acetaminophen is broken down through glucuronidation, sulfation, and the CYP2E1 enzyme – and these routes of detoxification don’t work the same in utero or even in premature babies.[ref]
Acetaminophen crosses the placental barrier and remains in an infant’s blood circulation for a longer duration. Animal studies show that unmetabolized acetaminophen may inhibit certain routes of brain development as well as fetal testosterone production.[ref]
Epidemiological studies show that there is an association between maternal acetaminophen use and ADHD rate. A study using cord blood metabolites as well as maternal acetaminophen use found that a higher acetaminophen burden was associated with a 2 to 3-fold increase in the risk of ADHD.[ref]
Is ADHD on the rise simply because it is overdiagnosed?
One question that frequently comes to mind for me is whether a condition that seems to be on the rise is being overdiagnosed – perhaps due to the availability and promotion of pharmaceuticals for the condition.
A scoping review of 334 research studies on ADHD concluded that overdiagnosis is common in children and adolescents. Broadening of the diagnostic criteria may be one reason for this. The researchers caution that long-term harm could be associated with diagnosing and treating ADHD in children with milder symptoms.[ref]
Studies with school-aged kids show that younger children in a class (late birthdays) are more likely to be diagnosed and medicated for ADHD compared to kids whose birthdays fall earlier in the school year.[ref] This raises the question of whether the younger kids are just not developmentally ready for the sit-down learning environment.
Does overdiagnosis account for the entire rise in ADHD cases? The same review of 334 studies found that ADHD diagnoses have steadily increased since 1989, and overdiagnosis was most likely not the sole cause.[ref]
ADHD Genotype Report:
Lifehacks: Natural solutions for ADHD
There are a ton of studies on ADHD that involve lifestyle changes, diet, circadian rhythm, or natural supplements. Please click through to the references for details on each of the studies. Talk with your doctor if you have questions or need medical advice.
Diet:
Does diet affect ADHD? Undoubtedly, an unhealthy diet can exacerbate symptoms.
Studies show that kids with ADHD are generally more likely to eat a less healthy diet.[ref] Overall, a healthy diet, higher in whole foods, should be a baseline to strive for when it comes to optimal cognitive health – for adults and children.
Studies also show that maternal diet is linked to ADHD in children. Mothers with particularly poor diets were more likely to have kids with ADHD.[ref]
Low histamine diet:
A low histamine diet may help with symptoms. Additionally, eliminating yellow and red food coloring may be worth a shot.
- Who Likely Benefits: Individuals with HNMT gene variants (rs1050891 A/A)
- Actions:
- Avoid high-histamine foods (e.g., aged cheeses, fermented products, processed meats, tomato sauce, spinach, chocolate, strawberries, etc.)
- Eliminate yellow and red food coloring
Related article: Histamine intolerance and a low-histamine diet.
Few-foods diet:
Related Articles and Topics:
Dopamine Receptor SNPs: Addiction, Mood, ADHD, and Schizophrenia
References:
Admin, Site. “Lithium and ADHD: How the Supplement Can Help.” Finally Focused, 11 Apr. 2022, https://finallyfocused.org/low-dose-lithium-adhd-supplement/.
Baek, Dae-Jung, et al. “Effect of Treadmill Exercise on Social Interaction and Tyrosine Hydroxylase Expression in the Attention-Deficit/ Hyperactivity Disorder Rats.” Journal of Exercise Rehabilitation, vol. 10, no. 5, Oct. 2014, pp. 252–57. PubMed, https://doi.org/10.12965/jer.140162.
Baird, A. L., et al. “Adult Attention-Deficit Hyperactivity Disorder Is Associated with Alterations in Circadian Rhythms at the Behavioural, Endocrine and Molecular Levels.” Molecular Psychiatry, vol. 17, no. 10, Oct. 2012, pp. 988–95. PubMed, https://doi.org/10.1038/mp.2011.149.
Becerra-Culqui, Tracy A., et al. “The Association of Prenatal Tetanus, Diphtheria, and Acellular Pertussis (Tdap) Vaccination With Attention-Deficit/Hyperactivity Disorder.” American Journal of Epidemiology, vol. 189, no. 10, Oct. 2020, pp. 1163–72. PubMed, https://doi.org/10.1093/aje/kwaa074.
Benedetti, F., et al. “Clock Genes beyond the Clock: CLOCK Genotype Biases Neural Correlates of Moral Valence Decision in Depressed Patients.” Genes, Brain, and Behavior, vol. 7, no. 1, Feb. 2008, pp. 20–25. PubMed, https://doi.org/10.1111/j.1601-183X.2007.00312.x.
Bilici, Mustafa, et al. “Double-Blind, Placebo-Controlled Study of Zinc Sulfate in the Treatment of Attention Deficit Hyperactivity Disorder.” Progress in Neuro-Psychopharmacology & Biological Psychiatry, vol. 28, no. 1, Jan. 2004, pp. 181–90. PubMed, https://doi.org/10.1016/j.pnpbp.2003.09.034.
Cabrera Lagunes, Alfonso, et al. “Association between CLOCK Gene Polymorphisms and ADHD in Mexican Teenagers: A Comprehensive Assessment.” Psychiatry Research, vol. 317, Nov. 2022, p. 114835. PubMed, https://doi.org/10.1016/j.psychres.2022.114835.
Dave, Usha Pinakin, et al. “An Open-Label Study to Elucidate the Effects of Standardized Bacopa Monnieri Extract in the Management of Symptoms of Attention-Deficit Hyperactivity Disorder in Children.” Advances in Mind-Body Medicine, vol. 28, no. 2, 2014, pp. 10–15.
Deepmala, null, et al. “Clinical Trials of N-Acetylcysteine in Psychiatry and Neurology: A Systematic Review.” Neuroscience and Biobehavioral Reviews, vol. 55, Aug. 2015, pp. 294–321. PubMed, https://doi.org/10.1016/j.neubiorev.2015.04.015.
Dorrego, María Flavia, et al. “A Randomized, Double-Blind, Crossover Study of Methylphenidate and Lithium in Adults with Attention-Deficit/Hyperactivity Disorder: Preliminary Findings.” The Journal of Neuropsychiatry and Clinical Neurosciences, vol. 14, no. 3, 2002, pp. 289–95. PubMed, https://doi.org/10.1176/jnp.14.3.289.
Dück, Alexander, et al. “Clock Genes Profiles as Diagnostic Tool in (Childhood) ADHD-A Pilot Study.” Brain Sciences, vol. 12, no. 9, Sept. 2022, p. 1198. PubMed, https://doi.org/10.3390/brainsci12091198.
Faltraco, Frank, et al. “Dopamine Adjusts the Circadian Gene Expression of Per2 and Per3 in Human Dermal Fibroblasts from ADHD Patients.” Journal of Neural Transmission (Vienna, Austria: 1996), vol. 128, no. 7, July 2021, pp. 1135–45. PubMed, https://doi.org/10.1007/s00702-021-02374-4.
Fargason, Rachel E., et al. “Correcting Delayed Circadian Phase with Bright Light Therapy Predicts Improvement in ADHD Symptoms: A Pilot Study.” Journal of Psychiatric Research, vol. 91, Aug. 2017, pp. 105–10. PubMed, https://doi.org/10.1016/j.jpsychires.2017.03.004.
Feng, Yu, et al. “Sequence Variation in the 3’-Untranslated Region of the Dopamine Transporter Gene and Attention-Deficit Hyperactivity Disorder (ADHD).” American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics, vol. 139B, no. 1, Nov. 2005, pp. 1–6. PubMed, https://doi.org/10.1002/ajmg.b.30190.
Fusar-Poli, Paolo, et al. “Striatal Dopamine Transporter Alterations in ADHD: Pathophysiology or Adaptation to Psychostimulants? A Meta-Analysis.” The American Journal of Psychiatry, vol. 169, no. 3, Mar. 2012, pp. 264–72. PubMed, https://doi.org/10.1176/appi.ajp.2011.11060940.
Geier, David A., et al. “A Cross-Sectional Study of the Relationship between Infant Thimerosal-Containing Hepatitis B Vaccine Exposure and Attention-Deficit/Hyperactivity Disorder.” Journal of Trace Elements in Medicine and Biology: Organ of the Society for Minerals and Trace Elements (GMS), vol. 46, Mar. 2018, pp. 1–9. PubMed, https://doi.org/10.1016/j.jtemb.2017.11.001.
Ginsberg, Ylva, et al. “Maternal Infection Requiring Hospitalization during Pregnancy and Attention-Deficit Hyperactivity Disorder in Offspring: A Quasi-Experimental Family-Based Study.” Journal of Child Psychology and Psychiatry, and Allied Disciplines, vol. 60, no. 2, Feb. 2019, pp. 160–68. PubMed, https://doi.org/10.1111/jcpp.12959.
Huang, Jian, et al. “Circadian Modulation of Dopamine Levels and Dopaminergic Neuron Development Contributes to Attention Deficiency and Hyperactive Behavior.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, vol. 35, no. 6, Feb. 2015, pp. 2572–87. PubMed, https://doi.org/10.1523/JNEUROSCI.2551-14.2015.
Huang, Lan, et al. “Maternal Smoking and Attention-Deficit/Hyperactivity Disorder in Offspring: A Meta-Analysis.” Pediatrics, vol. 141, no. 1, Jan. 2018, p. e20172465. PubMed, https://doi.org/10.1542/peds.2017-2465.
Huang, Yu-Hui, et al. “Significantly Lower Serum and Hair Magnesium Levels in Children with Attention Deficit Hyperactivity Disorder than Controls: A Systematic Review and Meta-Analysis.” Progress in Neuro-Psychopharmacology & Biological Psychiatry, vol. 90, Mar. 2019, pp. 134–41. PubMed, https://doi.org/10.1016/j.pnpbp.2018.11.012.
Huss, Michael, et al. “Supplementation of Polyunsaturated Fatty Acids, Magnesium and Zinc in Children Seeking Medical Advice for Attention-Deficit/Hyperactivity Problems – an Observational Cohort Study.” Lipids in Health and Disease, vol. 9, Sept. 2010, p. 105. PubMed, https://doi.org/10.1186/1476-511X-9-105.
Hwang, In Wook, et al. “Association of Monoamine Oxidase A (MAOA) Gene UVNTR and Rs6323 Polymorphisms with Attention Deficit and Hyperactivity Disorder in Korean Children.” Medicina (Kaunas, Lithuania), vol. 54, no. 3, May 2018, p. 32. PubMed, https://doi.org/10.3390/medicina54030032.
Katzenberg, D., et al. “A CLOCK Polymorphism Associated with Human Diurnal Preference.” Sleep, vol. 21, no. 6, Sept. 1998, pp. 569–76. PubMed, https://doi.org/10.1093/sleep/21.6.569.
Kerekes, Nóra, et al. “Neuroinflammation as a Possible Link between Attention-Deficit/Hyperactivity Disorder (ADHD) and Pain.” Medical Hypotheses, vol. 157, Dec. 2021, p. 110717. PubMed, https://doi.org/10.1016/j.mehy.2021.110717.
Kim, Eunjoo, et al. “The Relationship between the SNAP-25 Polymorphism and Omission Errors in Korean Children with Attention Deficit Hyperactivity Disorder.” Clinical Psychopharmacology and Neuroscience: The Official Scientific Journal of the Korean College of Neuropsychopharmacology, vol. 15, no. 3, Aug. 2017, pp. 222–28. PubMed, https://doi.org/10.9758/cpn.2017.15.3.222.
Larsson, Henrik, et al. “Childhood Attention-Deficit Hyperactivity Disorder as an Extreme of a Continuous Trait: A Quantitative Genetic Study of 8,500 Twin Pairs.” Journal of Child Psychology and Psychiatry, and Allied Disciplines, vol. 53, no. 1, Jan. 2012, pp. 73–80. PubMed, https://doi.org/10.1111/j.1469-7610.2011.02467.x.
Li, Yueling, et al. “Do SNPs of DRD4 Gene Predict Adult Persistence of ADHD in a Chinese Sample?” Psychiatry Research, vol. 205, no. 1–2, Jan. 2013, pp. 143–50. PubMed, https://doi.org/10.1016/j.psychres.2012.08.016.
Lo-Castro, Adriana, et al. “ADHD and Genetic Syndromes.” Brain & Development, vol. 33, no. 6, June 2011, pp. 456–61. PubMed, https://doi.org/10.1016/j.braindev.2010.05.011.
Nunes, Marielza Andrade, et al. “Microdose Lithium Treatment Stabilized Cognitive Impairment in Patients with Alzheimer’s Disease.” Current Alzheimer Research, vol. 10, no. 1, Jan. 2013, pp. 104–07. PubMed, https://doi.org/10.2174/1567205011310010014.
Pan, Yu-Qing, et al. “Association between ANKK1 (Rs1800497) Polymorphism of DRD2 Gene and Attention Deficit Hyperactivity Disorder: A Meta-Analysis.” Neuroscience Letters, vol. 590, Mar. 2015, pp. 101–05. PubMed, https://doi.org/10.1016/j.neulet.2015.01.076.
Serrano, Samantha E., et al. “Phthalates and Diet: A Review of the Food Monitoring and Epidemiology Data.” Environmental Health, vol. 13, no. 1, June 2014, p. 43. BioMed Central, https://doi.org/10.1186/1476-069X-13-43.
Sheehan, K., et al. “Tryptophan Hydroxylase 2 (TPH2) Gene Variants Associated with ADHD.” Molecular Psychiatry, vol. 10, no. 10, Oct. 2005, pp. 944–49. PubMed, https://doi.org/10.1038/sj.mp.4001698.
Starobrat-Hermelin, B., and T. Kozielec. “The Effects of Magnesium Physiological Supplementation on Hyperactivity in Children with Attention Deficit Hyperactivity Disorder (ADHD). Positive Response to Magnesium Oral Loading Test.” Magnesium Research, vol. 10, no. 2, June 1997, pp. 149–56.
Stevenson, Jim, et al. “The Role of Histamine Degradation Gene Polymorphisms in Moderating the Effects of Food Additives on Children’s ADHD Symptoms.” American Journal of Psychiatry, vol. 167, no. 9, Sept. 2010, pp. 1108–15. DOI.org (Crossref), https://doi.org/10.1176/appi.ajp.2010.09101529.
Surman, Craig, et al. “L-Threonic Acid Magnesium Salt Supplementation in ADHD: An Open-Label Pilot Study.” Journal of Dietary Supplements, vol. 18, no. 2, 2021, pp. 119–31. PubMed, https://doi.org/10.1080/19390211.2020.1731044.
Tsaltas, Eleftheria, et al. “Enhancing Effects of Chronic Lithium on Memory in the Rat.” Behavioural Brain Research, vol. 177, no. 1, Feb. 2007, pp. 51–60. PubMed, https://doi.org/10.1016/j.bbr.2006.11.003.
van Andel, Emma, et al. “Effects of Chronotherapy on Circadian Rhythm and ADHD Symptoms in Adults with Attention-Deficit/Hyperactivity Disorder and Delayed Sleep Phase Syndrome: A Randomized Clinical Trial.” Chronobiology International, vol. 38, no. 2, Feb. 2021, pp. 260–69. PubMed, https://doi.org/10.1080/07420528.2020.1835943.
Villemonteix, Thomas, et al. “Structural Correlates of COMT Val158Met Polymorphism in Childhood ADHD: A Voxel-Based Morphometry Study.” The World Journal of Biological Psychiatry: The Official Journal of the World Federation of Societies of Biological Psychiatry, vol. 16, no. 3, Apr. 2015, pp. 190–99. PubMed, https://doi.org/10.3109/15622975.2014.984629.
Wagner-Schuman, Melissa, et al. “Association of Pyrethroid Pesticide Exposure with Attention-Deficit/Hyperactivity Disorder in a Nationally Representative Sample of U.S. Children.” Environmental Health, vol. 14, no. 1, May 2015, p. 44. BioMed Central, https://doi.org/10.1186/s12940-015-0030-y.
Wang, Yanni, et al. “The Potential Role of Clock Genes in Children Attention-Deficit/Hyperactivity Disorder.” Sleep Medicine, vol. 71, July 2020, pp. 18–27. PubMed, https://doi.org/10.1016/j.sleep.2020.02.021.
Yoshikawa, Takeo, et al. “Histamine N-Methyltransferase in the Brain.” International Journal of Molecular Sciences, vol. 20, no. 3, Feb. 2019, p. 737. PubMed, https://doi.org/10.3390/ijms20030737.
Ziereis, Susanne, and Petra Jansen. “Effects of Physical Activity on Executive Function and Motor Performance in Children with ADHD.” Research in Developmental Disabilities, vol. 38, Mar. 2015, pp. 181–91. PubMed, https://doi.org/10.1016/j.ridd.2014.12.005.