Key takeaways:
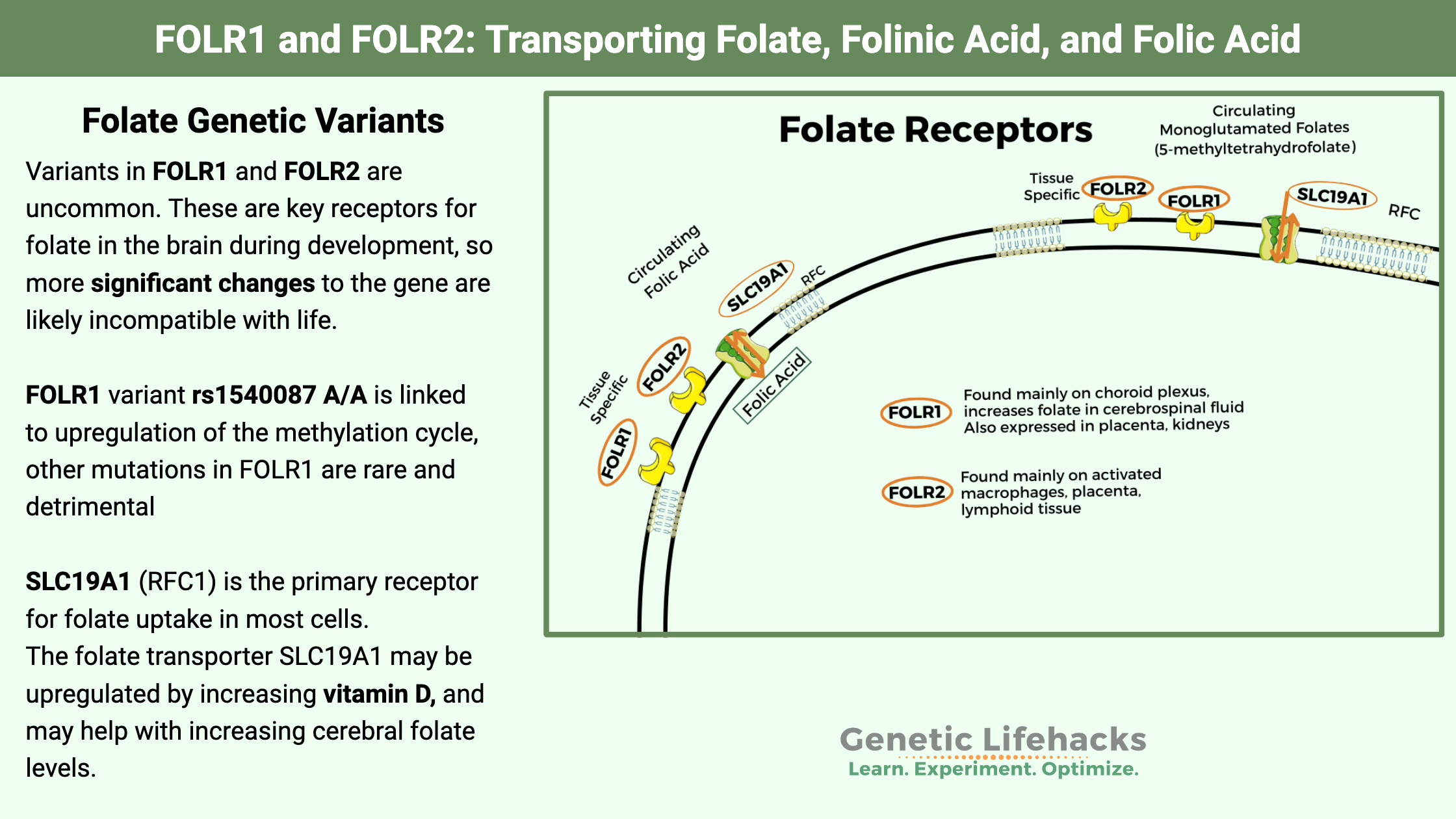
~ While the reduced folate carrier (SLC19A1) is the dominant folate receptor throughout the body, two other folate receptors enhance folate uptake in a few specific tissues.
~ The folate receptor alpha (FOLR1 gene) is important for the higher folate levels in the cerebrospinal fluid that provide the folate needed in the brain. FOLR2 is important in the immune response.
~ There are a couple of genetic variants and rare mutations that can affect the function of FOLR1 and FOLR2. In addition, antibodies against folate receptor alpha may contribute to cerebral folate deficiency.
Folate: Why cells need it an how they get it
Folate, or vitamin B9, is an essential nutrient used by cells for many different processes. When you eat foods that contain folate, natural folate is broken down and absorbed in your intestines and then circulates throughout your body. Folate is needed for the synthesis of DNA, RNA, and amino acids, which are the building blocks of proteins. Cells need a way to take up circulating folate, and there are several receptors that do this.
Bringing folate into the cell:
The main folate receptor found in almost all cell types is SLC19A1 (reduced folate carrier, also called RFC1). However, certain cell types have additional folate receptors that can influence the uptake of folate into tissues.
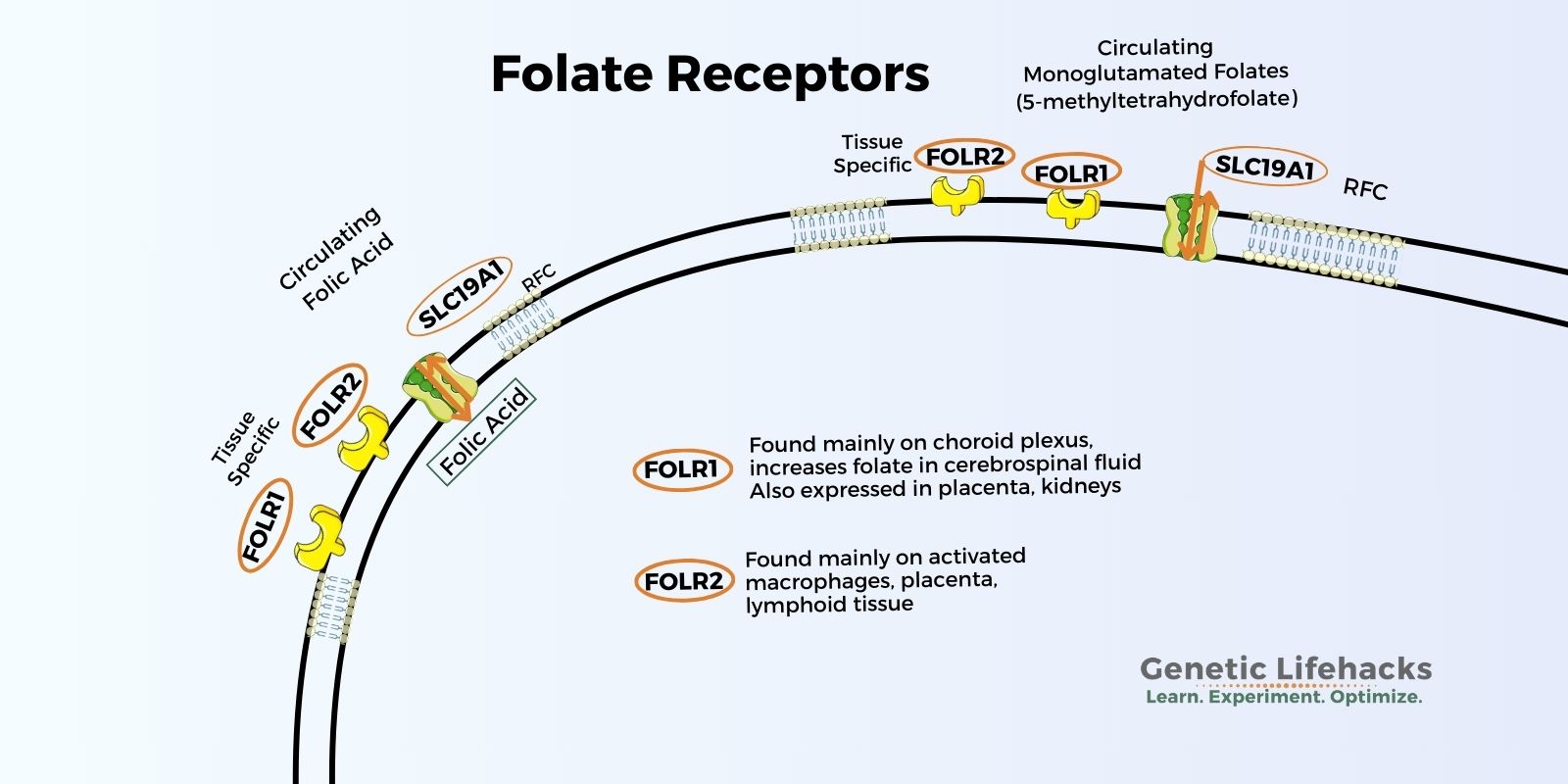
FOLR1 and FOLR2 are folate receptors expressed in certain tissues that increase the transport of folate into cells
| Receptor | Gene | Main tissues | Function |
|---|---|---|---|
| SLC19A1 | SLC19A1 | Most tissues | General folate transport |
| Folate receptor alpha (FRα) | FOLR1 | Placenta, brain, kidney, etc. | High-affinity uptake, fetal dev. |
| Folate receptor beta (FRβ) | FOLR2 | Immune organs, macrophages | Immune response, inflammation |
| FRγ/δ | FOLR3 | (Emerging data) | Regulatory T-cells (hypothesized) |
Let’s take a deeper look at the folate receptors and their functions.
Folate Receptor Alpha (FOLR1):
Expression and Role:
- Location: Placenta, choroid plexus, kidney, cancer cells
- Critical for fetal development and brain function
- Rare FOLR1 mutations cause cerebral folate deficiency
- Associated symptoms: epilepsy, ataxia, intellectual disability
FOLR1 (folate receptor 1) is the gene encoding folate receptor alpha (FRα). This protein is located on the surface of certain cells and plays a critical role in the uptake and regulation of folate for certain cell types. The FRα protein acts as a receptor that binds to folate and helps transport it into cells. FOLR1 is expressed in several tissues, including the placenta, choroid plexus (where cerebrospinal fluid is produced), and kidneys. It is also found in some cancer cells, most notably ovarian and lung cancers.
Fetal development:
Adequate folate uptake is critical for proper fetal development, particularly for the formation of the neural tube, which gives rise to the brain and spinal cord. The FRα protein plays an important role in this process by facilitating folate transport across the placenta to the developing fetus. FOLR1 can also be secreted as a folate-binding protein in breast milk and semen. In breast milk, this allows folate to be transported to the infant. [ref][ref]
Genetic variations:
Rare mutations in the FOLR1 gene can cause a rare genetic disorder called cerebral folate deficiency (CFD).[ref][ref]
Cancer treatment:
Because some cancer cells overexpress FRα, researchers are looking at ways to target this protein as a potential cancer therapy. One approach involves using antibodies or small molecules that specifically bind to FRα to deliver drugs or imaging agents directly to cancer cells.
Cerebral Folate Deficiency:
Folate is absorbed in the intestines and then circulates in the bloodstream. The bloodstream is separated from the brain and spinal cord to protect these vital organs from pathogens. Therefore, folate must be delivered via receptors to the choroid plexus, the network where cerebrospinal fluid is produced. The FOLR1 receptor is highly expressed on the choroid plexus, and folate concentrations in cerebrospinal fluid are typically about 1.5 times higher than in serum. [ref]
The SLC19A1 transporter (a.k.a. reduced folate carrier or RFC1) is also found on the choroid plexus for folate transport and is responsible for part of the folate moving into the brain. However, when FOLR1 is impaired by genetic mutations or folate receptor alpha antibodies, there is a reduction in cerebrospinal fluid folate levels because the reduced folate carrier can’t completely compensate for the lack of FOLR1.[ref]
Cerebral folate deficiency syndrome is a neurological syndrome or developmental disorder with low levels of methylfolate (5-MTHF) in the cerebrospinal fluid (CSF) but normal folate levels in the rest of the body. The syndrome may be caused by defective uptake of folate into the CSF due to problems with the FOLR1 receptor. Cerebral folate deficiency can cause neurological disorders such as epilepsy, ataxia, and intellectual disability.[ref][ref]
In addition to reduced folate transport (via FOLR1), other causes of cerebral folate deficiency syndrome include reduced folate storage, metabolic or mitochondrial problems, of increased folate utilization in the brain. Some children with infantile-onset cerebral folate deficiency develop low-IQ autism symptoms.[ref]
Adult-onset cerebral folate deficiency can be caused by mitochondrial diseases, liver disease, or brain calcifications. It can cause movement disorders, ataxia, pyramidal syndrome (spasms, contractions), and cognitive dysfunction. In some people, the condition can be reversed with folinic acid (Leucovorin). [ref][ref]
Folate receptor alpha and autism:
Antibodies against the folate receptor are suspected to play a role in autism, at least for some individuals. A meta-analysis of studies on cerebral folate deficiency found that its prevalence was 38% in autism spectrum disorder. Folate receptor alpha antibodies were thought to be the cause of cerebral folate deficiency in 83% of cases.[ref]
In children with cerebral folate deficiency and folate receptor antibodies, treatment with folinic acid (d,l-leucovorin) significantly improved autism symptoms.[ref]
Folate receptor beta (FOLR2):
Expression and Role
- Found in hematopoietic tissue and activated macrophages
- Overexpressed in some cancers and autoimmune diseases
The FOLR2 gene codes for folate receptor beta (FRβ). FOLR2 is primarily expressed in hematopoietic (blood-forming) tissues, such as the spleen, thymus, and bone marrow. FRβ is also found on the surface of activated macrophages, which are immune cells involved in the body’s defense against pathogens and in inflammation.
Inflammation, fibrosis, and autoimmunity:
Folate receptor beta (FOLR2) expression is upregulated in activated macrophages during inflammation and in some autoimmune diseases, such as rheumatoid arthritis. Activated M2 macrophages increase fibrosis and growth by secreting lots of TGF-beta, and researchers are looking at blocking FOLR2 in autoimmune diseases to reduce macrophage activity.[ref]
Cancer:
Like FRα, FRβ is also overexpressed in some cancer cells, particularly in myeloid leukemia and in tumor-associated macrophages (TAMs). TAMs are macrophages that are recruited to the tumor microenvironment and can promote tumor growth, angiogenesis (blood vessel formation), and metastasis. Overexpression of FRβ in these cells makes it a potential target for cancer therapy.[ref]
Genetic disorders:
While there are some rare mutations in FOLR1 associated with cerebral folate deficiency, there are currently no known genetic disorders specifically linked to mutations in the FOLR2 gene.
FOLR2 and PFAS (forever chemicals):
Animal studies show that certain types of PFAS interact with FOLR2 (folate receptor beta) and cause social deficits.[ref] A 2024 study in Shanghai found that prenatal PFOA exposure increased autistic traits in children at the age of 4 in those with genetic risk factors. The risk was mitigated by higher folate intake.[ref]
Related article: PFAS, Your Genes, and Your Health
Genotype report: FOLR1 and FOLR2
Mutations and SNPs in FOLR1 and FOLR2 are uncommon. These are key receptors for folate in the brain during development, so more significant changes to the gene are likely incompatible with life.[ref] FOLR1 gene: encodes the folate receptor alpha, which is a cell receptor that transports in folate for certain cell types.
Check your genetic data for rs144637717 (23andMe v4, v5; AncestryDNA):
- T/T: typical
- C/T: cerebral folate deficiency possible[ref], especially in conjunction with a second mutation
- C/C: cerebral folate deficiency possible[ref]
Members: Your genotype for rs144637717 is —.
Check your genetic data for rs1540087 (AncestryDNA):
- G/G: typical
- A/G: lower odds of skin lesions with arsenic exposure (methyl groups are utilized for arsenic detoxification, better methylation cycle)
- A/A: lower odds of skin lesions with arsenic exposure (methyl groups are utilized for arsenic detoxification, better methylation cycle)[ref] (good)
Members: Your genotype for rs1540087 is —.
Check your genetic data for rs2071010 (23andMe v4, AncestryDNA):
- A/A: slightly increased relative risk of congenital heart disease in offspring[ref][ref] increased relative risk of high homocysteine[ref]
- A/G: typical risk
- G/G: typical
Members: Your genotype for rs2071010 is —.
FOLR2 gene:
Check your genetic data for rs514933 (AncestryDNA):
- C/C: reduced relative risk of congenital heart disease in offspring[ref] (good)
- C/T: reduced relative risk of congenital heart disease in offspring
- T/T: typical
Members: Your genotype for rs514933 is —.
Are there other folate receptors?
Yes. There is also Folate Receptor-gamma (FRγ) and FR-delta (FRδ, FOLR3), but their function isn’t fully known or understood – yet. Folate receptor-delta is likely important in regulatory T-cells.[ref]
Lifehacks:
Track: If you have a folate receptor variant, look at how much folate you get from natural foods containing folate as well as how much folic acid you get from fortified foods. Cronometer.com is a free web app that lets you track your nutrient intake from foods that you eat. You may be surprised at how much (or little) folate you consume.
Caution with high doses: While there are specific reasons for high doses of folate supplements, such as cerebral folate deficiency, the flip side is the use of folate by cancerous cells. In my opinion, folate supplements should be approached with some caution in people who are at risk for cancer. Talk with your doctor if you have any questions about supplements.
Access this content:
An active subscription is required to access this content.
Related articles and topics:
Homocysteine: Genetics, High Homocysteine Levels, and Solutions
References: