Key takeaways:
- IL-2 has a dual role in the immune system, preventing autoimmunity at low levels and fighting pathogens and cancer at higher levels.
- The IL-2 receptor determines the immune response. Different receptor combinations send different signals into your cells.
- Elevated soluble IL-2RA levels are found in long COVID patients with persistent fatigue. Low IL-2 contributes to autoimmune diseases like lupus and multiple sclerosis.
- Genetic variants in the IL2 genes influence your risk for autoimmune diseases, certain cancers, and how well your immune system fights infections.
- Natural compounds can modulate IL-2 signaling.
Interleukin-2: Immune response
Interleukin-2 (IL-2) is a cytokine protein that has many different roles in the immune response. It is important in the inflammatory response that fights off infectious diseases, and it also has an immune system modulatory role that dampens the immune response (important for preventing autoimmune attacks).
IL-2’s function—whether pro-inflammatory (fighting infections and cancer) or immunomodulatory (preventing autoimmune disease)—depends both on the tissues where it is activated and on the receptors that it binds to.
Let’s look at the background science on IL-2 and the IL-2 receptor first, and then go into how this could affect long Covid, autoimmune diseases, and cancer therapy response. Then we will dive into your genetic variants that increase or decrease IL-2 response.
Interleukin 2: What does it do?
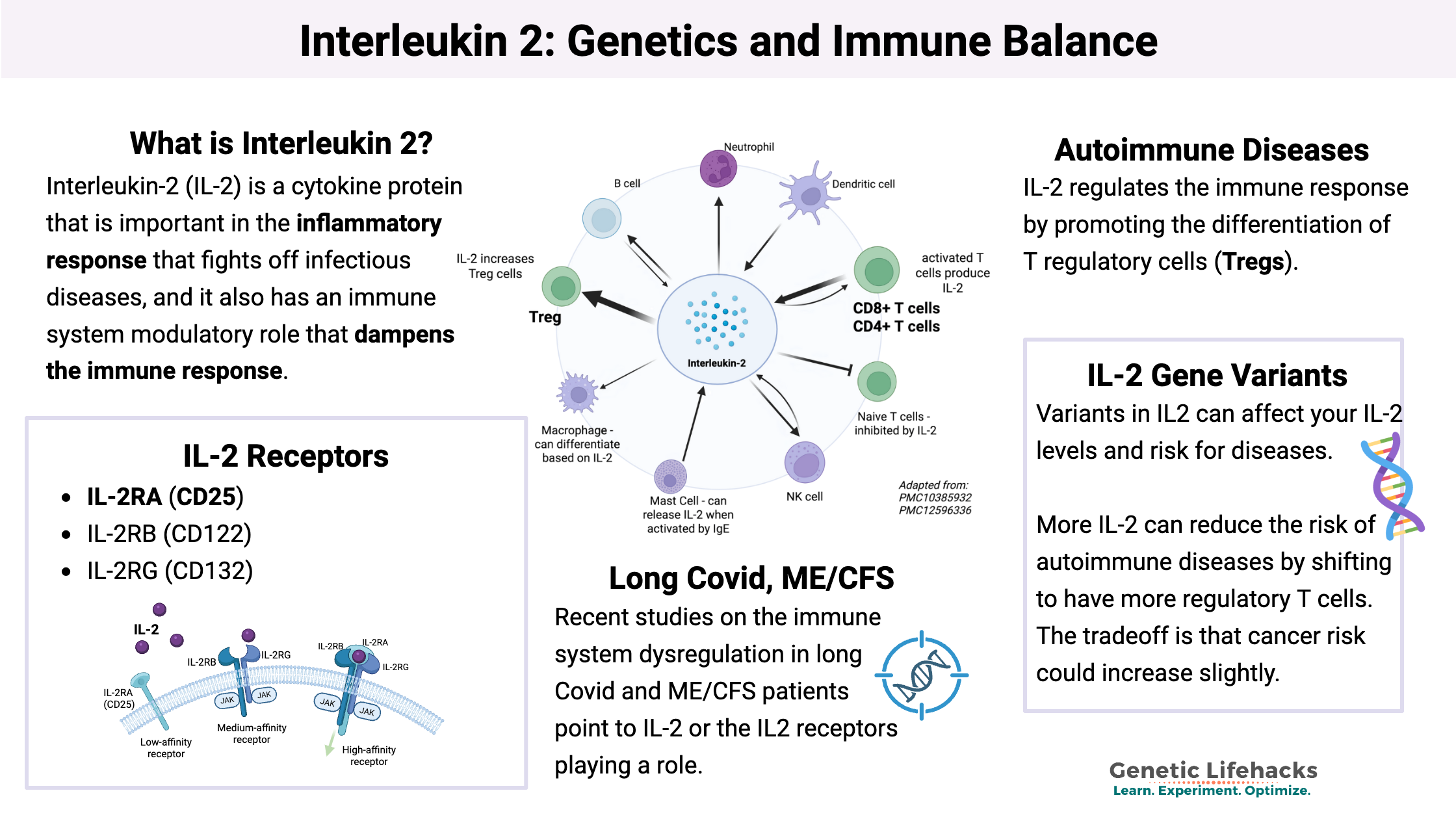
IL-2 is a cytokine in the immune system that regulates the activities of different types of white blood cells, called T cells.
- Activated T helper cells (CD4+) and killer T cells (CD8+) synthesize and release IL-2 in order to signal for the stimulation of other types of T cells and B cells.
- T regulatory (T reg) cells are also activated by low levels of IL-2.
Thus, IL-2 plays a role in both activating and modulating the immune response, making it important in fighting cancer and pathogens as well as maintaining immune balance.[ref][ref]
Here’s an example: IL-2 is involved in the CD8+ T cell response against viral infections. Without sufficient IL-2, the CD8+ T cell response to viral infection is about threefold lower.[ref]
In addition to T cells, other immune system cells, like mast cells, natural killer cells, and B cells, can also produce IL-2.[ref][ref]
IL-2 receptors receive the signal:
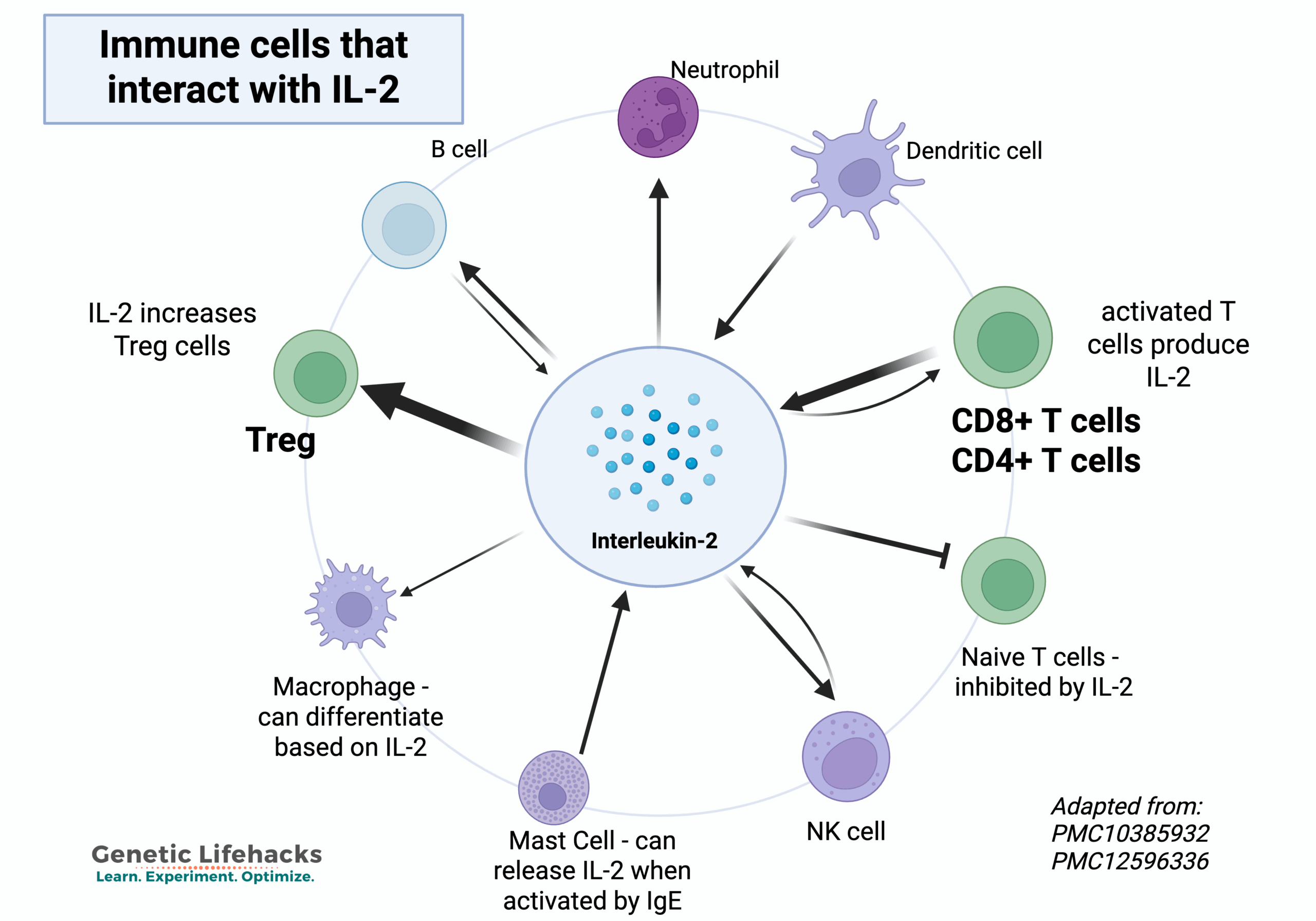
IL-2 exerts its effects by binding to the IL-2 receptor (IL-2R), to send its signal and cause changes in other immune system cells.
There are three IL-2 receptors that determine the immune response. Which receptor subunits IL-2 binds to matters just as much as IL-2 levels themselves. Different receptor combinations send different signals into your cells.
The three IL-2 receptors are:
- IL-2RA (Interleukin 2 receptor alpha, CD25)
- IL-2RB (Interleukin 2 receptor beta, CD122)
- IL-2RG (Interleukin 2 receptor gamma, CD132).
When IL-2 binds to its receptors, it sends a signal into the cell to have certain genes transcribed into proteins.
- At low levels, IL-2 helps regulate the immune response and may help prevent autoimmune attacks. [ref]
- At higher levels, IL-2 stimulates an anti-tumor immune response.
The three different receptor subunits determine the nature of the immune response.[ref]
- IL-2RA: IL-2 can bind easily to IL-2RA, but this receptor binding alone doesn’t send a signal into the cells.
- IL-2RB plus IL-2RG: Binding to these two receptor units, IL-2 signals for T cell growth and other immune responses.
- IL-2RA, IL-2RB, and IL-2RG: When all three subunits together bind with IL-2, this drives a stronger cellular response. The three receptors together are found on activated T cells and on T regulatory cells.
Soluble IL-2R (sIL-2R):
The receptor for IL-2 can be shed from the surface of activated T cells or B cells. This is known as soluble IL-2RA (sIL-2Rα or sCD25) — the detached IL-2 receptor that normally is on immune cells. It’s a marker of lymphocyte activation and immune system dysfunction. Elevated levels are used to diagnose and monitor autoimmune disorders, lymphomas, and infections.[ref]
IL-2 in Autoimmune Diseases:
IL-2 regulates the immune response, important in autoimmune diseases, by promoting the differentiation of T regulatory cells (Tregs). Tregs are specialized T cells that act to maintain self-tolerance and suppress excessive immune responses. They are defined by having the markers CD4+, CD25, and FOXP3+. They prevent autoimmunity and limit chronic inflammation, but they can also be a problem for the anti-tumor activity of other T cells.[ref]
Balance is important here. Low levels of IL-2 activate regulatory T cells, which keep the immune response in balance.[ref] Mice that are bred to be deficient in IL-2 or the IL-2 receptor will quickly develop autoimmune problems due to a lack of T reg cells.
As an example, in lupus, researchers have found aberrant IL-17A expression along with reduced IL-2 production, decreasing Treg production. This is due, at least in part, because high SIRT2 levels in lupus patients suppress IL-2. Genetic studies also show that IL-2 plays a causal role in myasthenia gravis.[ref][ref]
Low-dose IL-2 is currently injected as a treatment for certain autoimmune diseases. However, researchers learned in the 1990s through cancer trials that giving people a high dose of IL-2 (immunotherapy) may cure some cancers, but for many, it induces toxicity and a cytokine storm.[ref]
IL-2 in Cancer Therapy:
IL-2, as an activator of T cells, plays an important role in the way the body naturally fights off cancer cells.
First cancer immunotherapy:
IL-2-based immunotherapy drugs for cancer were first used experimentally in the 1980s, and these recombinant IL-2 drugs were the first forms of cancer immunotherapy. However, significant toxic effects were also experienced with these drugs.
Recombinant IL-2 (aldesleukin) was FDA-approved in 1998 for melanoma and advanced renal cell carcinoma, but it only worked for a portion of patients. Over the last three decades, IL-2 therapies have evolved to be used alongside other therapies, but usually as a second-tier option if immune checkpoint inhibitors fail. [ref][ref]
In cancer, IL-2 can be beneficial to drive a strong immune response against tumor cells. However, this is balanced by the activation of Tregs, which then dampen the anti-tumor response. The problem that researchers have run into with targeting IL-2 or IL-2R in cancer immunotherapy is that there are so many parts of the immune response that IL-2 targets that there are off-target adverse effects, like vascular leak syndrome.[ref]
IL-2 in Long Covid, ME/CFS:
Recent studies on the immune system dysregulation in long Covid and ME/CFS patients point to IL-2 or the IL2 receptors playing a role. Some of these studies suggest persistent T cell activation with high IL-2RA levels, which then leads to T cell exhaustion along with ongoing elevated cytokines (including IL-2). However, not all of the studies agree — pointing to a possibility of multiple subtypes of both long Covid and ME/CFS.
- A 2025 study that followed long Covid patients for more than 18 months found that patients with persistent fatigue and shortness of breath at 6-18 months had significantly higher levels of soluble IL2RA (CD25). [ref]
- Another 2025 study also found up to 4-fold higher IL2RA (CD25) expression on CD8+ T cells in long Covid patients up to 18 months after infection. The conclusion was that there is a long-lasting CD8+ T cell hyperactivation in long Covid.[ref]
- Other studies show high levels of circulating IL-2, indicating long-term activation, in long Covid.[ref]
Dysregulation of IL-2 is also reported in ME/CFS (chronic fatigue) patients.
- IL-2 is downregulated, along with other markers for CD8+ T cell exhaustion, in some ME/CFS patients. The researchers also found differences between Tregs in ME/CFS compared to long Covid.[ref]
- However, other studies show IL2 is significantly higher in extracellular vesicles in ME/CFS patients.[ref]
Genotype report: IL-2
Access this content:
An active subscription is required to access this content.
Lifehacks: Evidence-Based Interventions
Let’s take a look at the ways that natural supplements and your diet can affect your IL-2 levels.
Dietary interactions:
Gene X Diet interaction:
A genetic study found that people with rs2069762 C alleles who also ate more soy had about half the risk of gastric cancer, compared to those with a lower intake of soy.[ref]
Ketogenic diet:
A study involving psoriasis patients showed that a ketogenic diet for 4 weeks caused a decrease in IL-2 concentrations.[ref]
For reducing elevated IL-2 or soluble IL2RA:
Several natural supplements and vitamins have been shown in studies to affect IL-2 levels and reduce the inflammatory response from IL-2.
Curcumin, at higher doses, blocks IL2 receptor binding:
Curcumin is a natural compound found in turmeric, and it is well known for its anti-inflammatory effects. A 2018 study showed that curcumin is a direct inhibitor of IL2 binding to its receptor (IL2RA). This inhibition is seen at high doses, such as those found in supplements, and not from low doses, such as from the spice turmeric. Some research points towards low doses having more of an immunomodulatory effect.[ref][ref][ref]
EGCG in green tea:
A polyphenol found in green tea, EGCG, also causes IL-2R signaling impairment. Studies show that EGCG doesn’t directly inhibit IL-2, but rather decreases receptor signaling, which then feedsback to decrease IL2 while also increasing T regs. [ref][ref] The delayed effect may mean that EGCG will take time to decrease excess IL-2 and systemic inflammation. In the cell line study, it took 48 hours for IL2 levels to be affected.
Vitamin D:
Vitamin D acts to moderate IL-2 levels in inflammatory conditions. It doesn’t reduce IL2 receptor binding, but instead, vitamin D acts on other regulators to suppress excess IL-2 and promote homeostasis. [ref][ref]
Riboflavin:
A study in patients with intestinal inflammation in Crohn’s disease showed that 100 mg/day of riboflavin decreased IL-2 levels.[ref]
For increasing or supporting IL-2 in immunodeficiency:
If you have low IL-2, T cell exhaustion, or immunodeficiency and want to boost IL-2, research shows:
Resveratrol boosts IL-2:
In animal studies of IL-2 cancer therapy, the addition of resveratrol is synergistic and helps to prevent the negative effects of high-dose IL-2. Some studies point to resveratrol increasing NK cell activity and allowing IL-2 immunotherapy in cancer to work at lower doses.[ref][ref]
Cordyceps mushroom extract:
A 2025 clinical trial showed that Cordyceps militaris mushroom extract stimulates natural killer cell activity and increases IL-2 signaling.[ref]
Get sufficient zinc:
One way that zinc is involved in boosting immune response is through increasing IL-2 signaling.[ref] Supplementing with zinc when deficient may help to increase IL-2 and T cell response. The US RDA for zinc is 8 – 11mg/day. Foods high in zinc include oysters, crab, pumpkin seeds, beef, and lamb.
Related articles and topics:
T Cell Exhaustion in Long COVID, ME/CFS, and Cancer: Mechanisms and Solutions
Interleukin-15 (IL-15): Immune response, cancer, long Covid, and genetics
IL-17: Chronic Inflammation & Autoimmune Risk with IL17A, IL17F