Key takeaways:
~ VMAT2 is nicknamed the “God gene” due to research studies linking it to spirituality.
~ VMAT2 packages up and releases neurotransmitters from neurons, regulating and facilitating neuronal transmission.
~ Genetic variants in VMAT2 alter the risk of alcohol dependence, Parkinson’s, and PTSD.
What is the “God” (VMAT2) gene?
Humans have long sought to integrate spirituality and science – wondering how consciousness, thought, feelings, self-transcendence, and spirituality are created in the brain. Genetics researchers have tried to answer these questions over the past few decades, and their search for a genetic basis for spirituality is intriguing.
VMAT2 is a neurotransmitter transporter encoded by the SLC18A2 (solute carrier family 18 member A2) gene.
The God Gene:
VMAT2 has been dubbed the “God gene” due to its reported association with spirituality.[ref] In a 2004 book by Dean Hammer, the VMAT2 gene was hypothesized to be the hereditary or genetic influence on spirituality. The book, called The God Gene: How Faith is Hardwired into our Genes, laid out the idea that spirituality is heritable and, at least partly, due to the VMAT2 gene.
The author suggests that positive selection for feel-good genes creates optimism for living even though death is inevitable.[ref]
What does the VMAT2 gene do?
VMAT2 (SLC18A2 gene) is a monoamine transport gene. It packages monoamine neurotransmitters from the cytosol inside of the neuron into vesicles to be released into the synapse.
Monoamine neurotransmitters include:
- dopamine
- serotonin
- adrenaline
- noradrenaline
- histamine
- melatonin
These are the neurotransmitters important in thinking, behavior, physical movement, pain, emotion, wakefulness, and circulation.[ref][ref]
Learn more about neurotransmitters: dopamine receptors, dopamine synthesis genes, serotonin genes, histamine genes, and COMT.
VMAT2 is important for transporting dopamine, serotonin, and other neurotransmitters into vesicles that are then released in the synapses of the neurons. In the cytosol of the neuronal cell, VMAT2 facilitates the packaging of the monoamines into secretory vesicles. These vesicles then move to the cell membrane to release their neurotransmitter payload into the synapse to send a signal to the next neuron.[ref]
This packaging up of the monoamine neurotransmitters is essential because monoamines are prone to oxidation in the cell. Thus, VMAT2 protects cells from oxidative stress from oxidized monoamines.[ref]
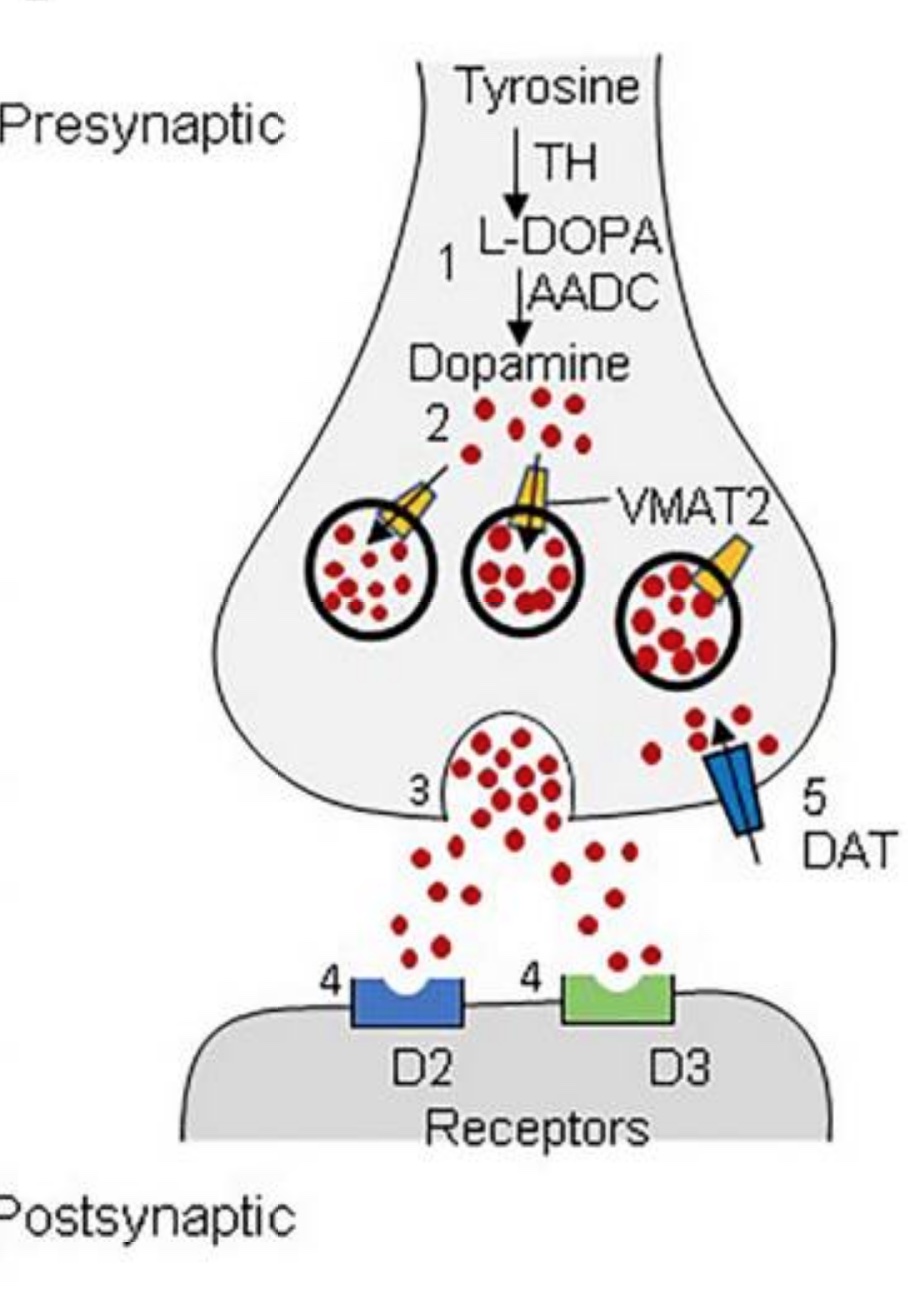
With its role in protecting neurons from oxidative stress, VMAT2 prevents damage to dopaminergic neurons. Parkinson’s results from damage to the dopaminergic neurons in the brain, and type 2 diabetes can result from dopamine damage in the pancreas. Newly developed VMAT-2 inhibitors have a possible side effect of causing Parkinson’s disease.[ref]
Research studies on VMAT-2:
For more than three decades, researchers have probed the functions of VMAT2. Here is a quick overview of recent research:
Parkinson’s:
Faulty dopaminergic transmission is at the root of Parkinson’s. Increased VMAT2 is protective against Parkinson’s disease and also protects against toxicants that can cause Parkinson’s.[ref]
VMAT2 essential for dopamine, serotonin:
Animal studies show that conditionally reducing VMAT2 expression causes a whole-brain decrease in monoamine neurotransmitters.[ref]
Protective against meth:
Increased levels of VMAT2 can protect the brain from damage from methamphetamine.[ref]
Protective against anxiety/depression:
Animal studies also showed that increased VMAT2 is protective against anxiety and depression. Additionally, increased VMTA2 enhances locomotion.[ref]
Protective against diabetes:
Another place VMAT2 is essential is in the beta-cells of the pancreas. Dopamine modulates the release of insulin, and VMAT2 is important here in the packaging up of dopamine. Thus, VMAT2 protects against oxidative stress in beta cells by controlling dopamine release.[ref]
VMAT2 Genotype Report
Access this content:
An active subscription is required to access this content.
Lifehacks: Altering VMAT2 Levels
VMAT2 inhibitors:
Inhibiting VMAT2 too much can cause Parkinson’s-like symptoms due to its effect on dopamine. The positive side of VMAT2 inhibitors is that they theoretically may help with addiction treatment for cocaine or meth.
Access this content:
An active subscription is required to access this content.
Related Articles and Topics:
Serotonin 2A receptor: Psychedelic response and Alzheimer’s disease
References: