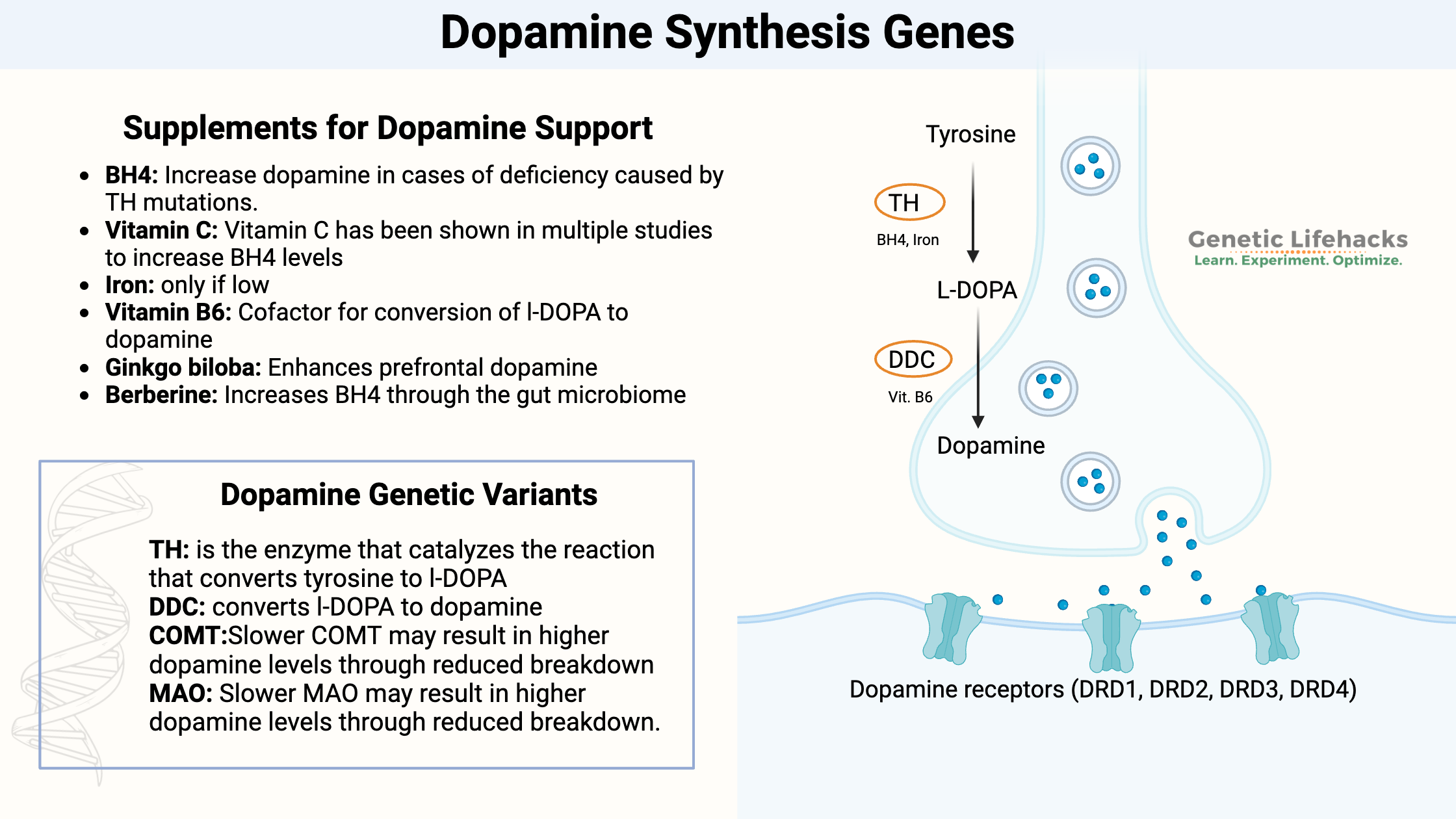
Key takeaways:
~ Dopamine synthesis begins with the conversion of tyrosine to l-DOPA, which is then converted to dopamine.
~ The key enzymes in this synthesis are TH (tyrosine hydroxylase) and DDC.
~ Genetic variants in these genes, along with lifestyle, diet, and other environmental factors, affect dopamine levels.
~ Testing can show you where you’re at, and supplements or dietary changes can help optimize dopamine levels.
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Consider joining today.
What does dopamine do?
Dopamine is a neurotransmitter that is essential for many brain functions and behaviors. It is the neurotransmitter that makes you feel good — pleasure, satisfaction, motivation…
Dopamine is part of the brain’s reward system, which reinforces pleasurable experiences and motivates you to do them again. In the part of the brain that controls movement, dopamine helps regulate motor control of voluntary muscles and coordination.
Dopamine is involved in attention, helping you focus and filter out irrelevant information. It helps form new memories, reinforces learning, and plays a role in evaluating outcomes and choosing appropriate actions.
Dopamine helps regulate emotions, especially positive feelings such as happiness and pleasure. Too little dopamine can make you feel moody, forgetful, unmotivated, etc. Too much dopamine, on the other hand, can cause someone to act without thinking things through.
The brain is all about balance with dopamine.
Let’s go into the details of how dopamine is made, then look at your genes for the dopamine pathway, and finish with ways to test and optimize dopamine levels.
Dopamine Synthesis Pathway:
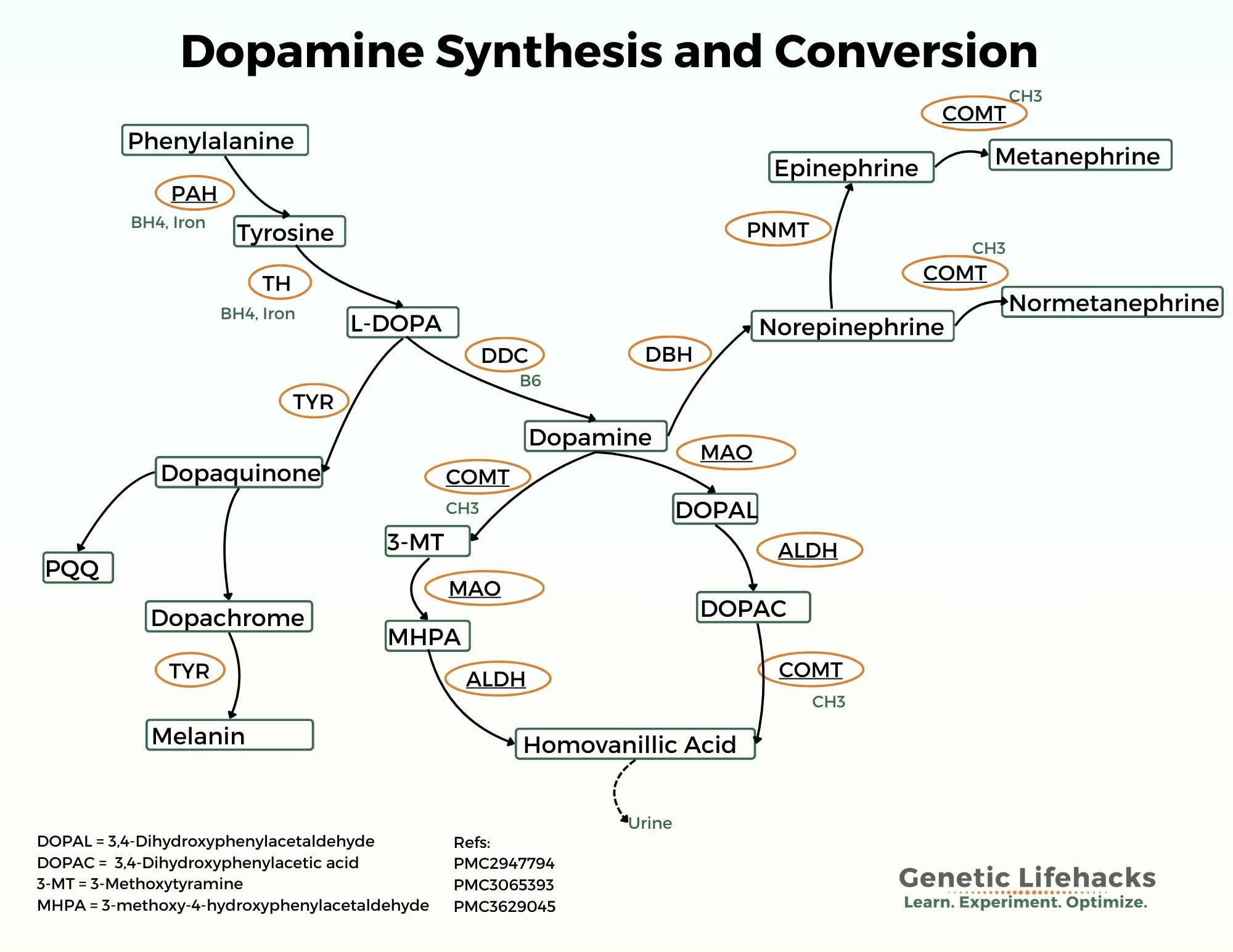
Dopamine is synthesized in neurons through a multistep process involving amino acids, enzymes, and specific cofactors.[ref]
The dopamine synthesis pathway begins with the conversion of l-tyrosine to l-DOPA. The enzyme tyrosine hydroxylase (TH gene) is used in the conversion. Tyrosine can be obtained from food or synthesized in the body from another amino acid, phenylalanine. Tetrahydrobiopterin (BH4) is required for the conversion of tyrosine to l-DOPA (and also for the conversion of phenylalanine to tyrosine). [ref]
l-Tyrosine —> l-DOPA
Next, l-DOPA (l-3,4-dihydroxyphenylalanine) is converted to dopamine using the enzyme aromatic amino acid decarboxylase (also called dopa decarboxylase, DDC gene).
l-DOPA —> Dopamine
The newly synthesized dopamine is then packaged into vesicles in the neuron so that it can be released. VMAT2 (vesicular monoamine transporter 2) is essential for the packaging of dopamine into vesicles.
Vesicles (VMAT2) —> Released into the synapse
Once released, dopamine binds to various dopamine receptors on the postsynaptic neuron. Dopamine is inactivated by reuptake into the presynaptic neuron via DAT (dopamine transporter) and is then metabolized by COMT and MAO (monoamine oxidase).
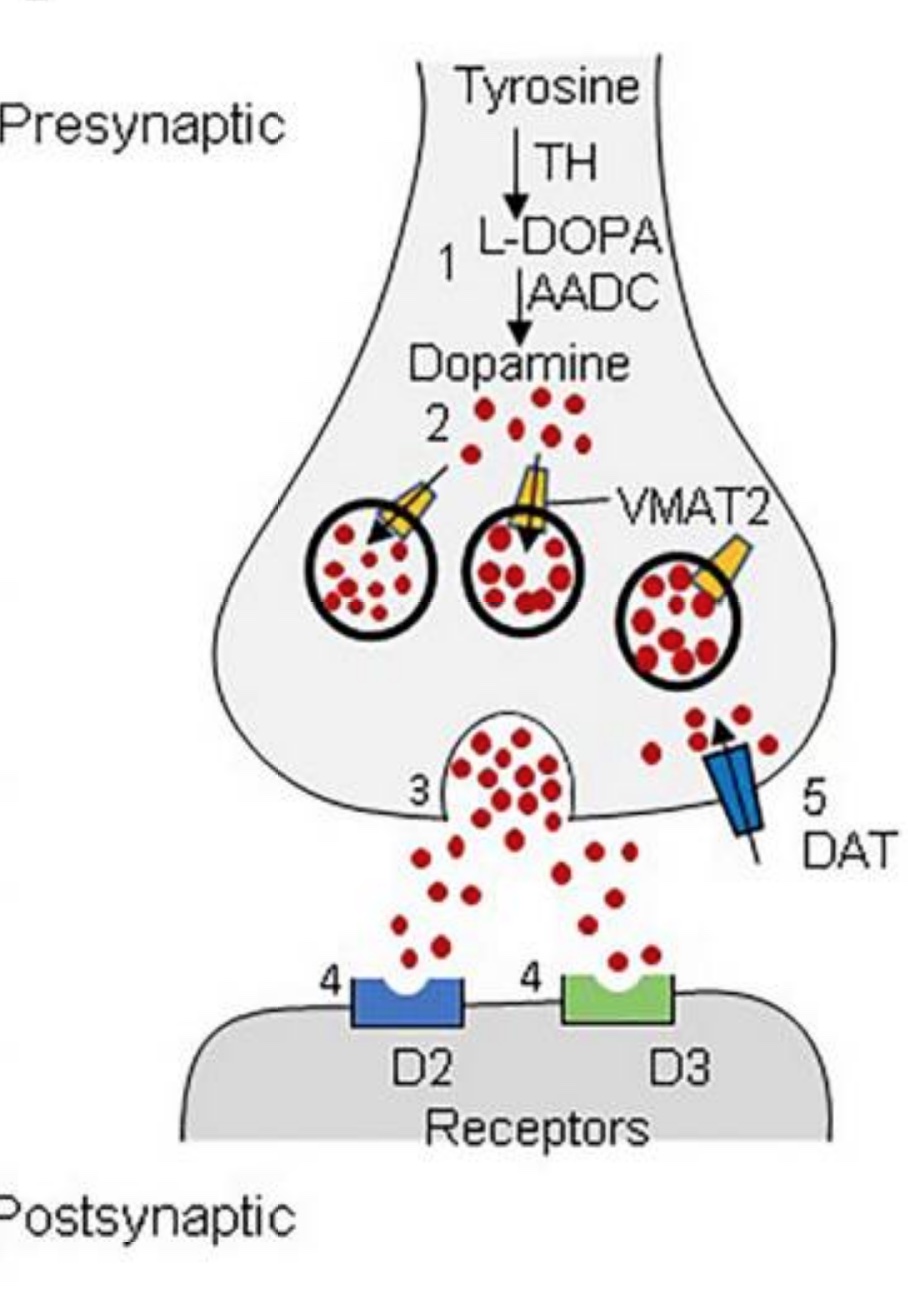
Tyrosine hydroxylase in-depth:
Tyrosine hydroxylase (TH) is the enzyme that catalyzes the reaction that converts tyrosine to l-DOPA. It is the rate-limiting step in dopamine production — meaning that TH is key to how much dopamine is produced.[ref] (The conversion of l-DOPA to dopamine is not normally limited.)
Regulation of tyrosine hydroxylase is tightly controlled in cells. When more dopamine, norepinephrine, or epinephrine is needed, TH is activated. Conversely, when less of these neurotransmitters are required, TH is inhibited through feedback mechanisms. The cell (neuron) controls this through the translation of the TH gene as well as post-translational modifications. Essentially, high levels of dopamine, norepinephrine, or epinephrine will all decrease TH levels by feedback inhibition.[ref]
What about genetic variants? While genetic variants in the TH gene are associated with slightly higher or lower dopamine levels, the whole system is still tightly regulated to keep dopamine levels in check. Phosphorylation, post-translational changes, feedback inhibition, and even other genes are involved in dopamine regulation.[ref]
One example of a gene that influences dopamine levels is PINK1 (PTEN induced putative kinase 1), which has been shown in studies to regulate tyrosine hydroxylase levels. That’s not necessarily a good thing. Rare mutations in PINK1 are associated with familial early-onset Parkinson’s disease. [ref][ref]
DOPA Decarboxylase (DDC) in-depth:
DOPA decarboxylase (DDC gene, also called aromatic L-amino acid decarboxylase) is the enzyme that facilitates the synthesis of dopamine from l-DOPA. It is a multifunctional enzyme that also synthesizes serotonin from 5-HTP. DDC is also involved in the synthesis of norepinephrine and several trace biogenic amines. It requires vitamin B6 as a cofactor.
l-DOPA —> dopamine
5-HTP —> serotonin
DDC is essential for life; severe deficiency-causing mutations are very rare and result in significant cognitive, motor, and autonomic impairments from birth. Common genetic variants in DDC can slightly alter its function (see the genotype report below). [ref][ref]
Packaging and transporting dopamine:
Once dopamine is synthesized in the neuron, it has to be packaged up and exported through the synapse in order to cause an effect.
This is where VMAT2 (also known as the God gene) comes into play. VMAT2 (SLC18A2 gene) is a monoamine transport gene. It packages monoamine neurotransmitters (dopamine, norepinephrine, epinephrine) from the cytosol into vesicles to be released by the neuron.
In this way, VMAT2 also regulates dopamine levels. Inhibiting VMAT2 results in higher dopamine levels within the neuron, which feeds back to inhibit tyrosine hydroxylase. [ref]
Related article: VMAT2 gene and neurotransmitter levels
Additional points to consider:
Again, neurotransmitter levels are complex. It is not as simple as more dopamine is good, although more dopamine will probably make you feel good initially.
One thing to keep in mind is that dopamine is the precursor to norepinephrine and epinephrine. So higher or lower dopamine levels can mean higher or lower levels of norepinephrine or epinephrine.
Dopamine levels vary over the course of 24 hours in a circadian rhythm controlled by the CLOCK gene, which is a core circadian rhythm gene. The CLOCK gene controls transcriptional activation of TH, and disruption of the CLOCK gene in animal studies causes disruption to dopamine levels (and changes to cocaine addiction).[ref] In addition to being controlled by circadian rhythm genes, dopamine may also be important in modulating circadian rhythm in different regions of the brain.[ref]
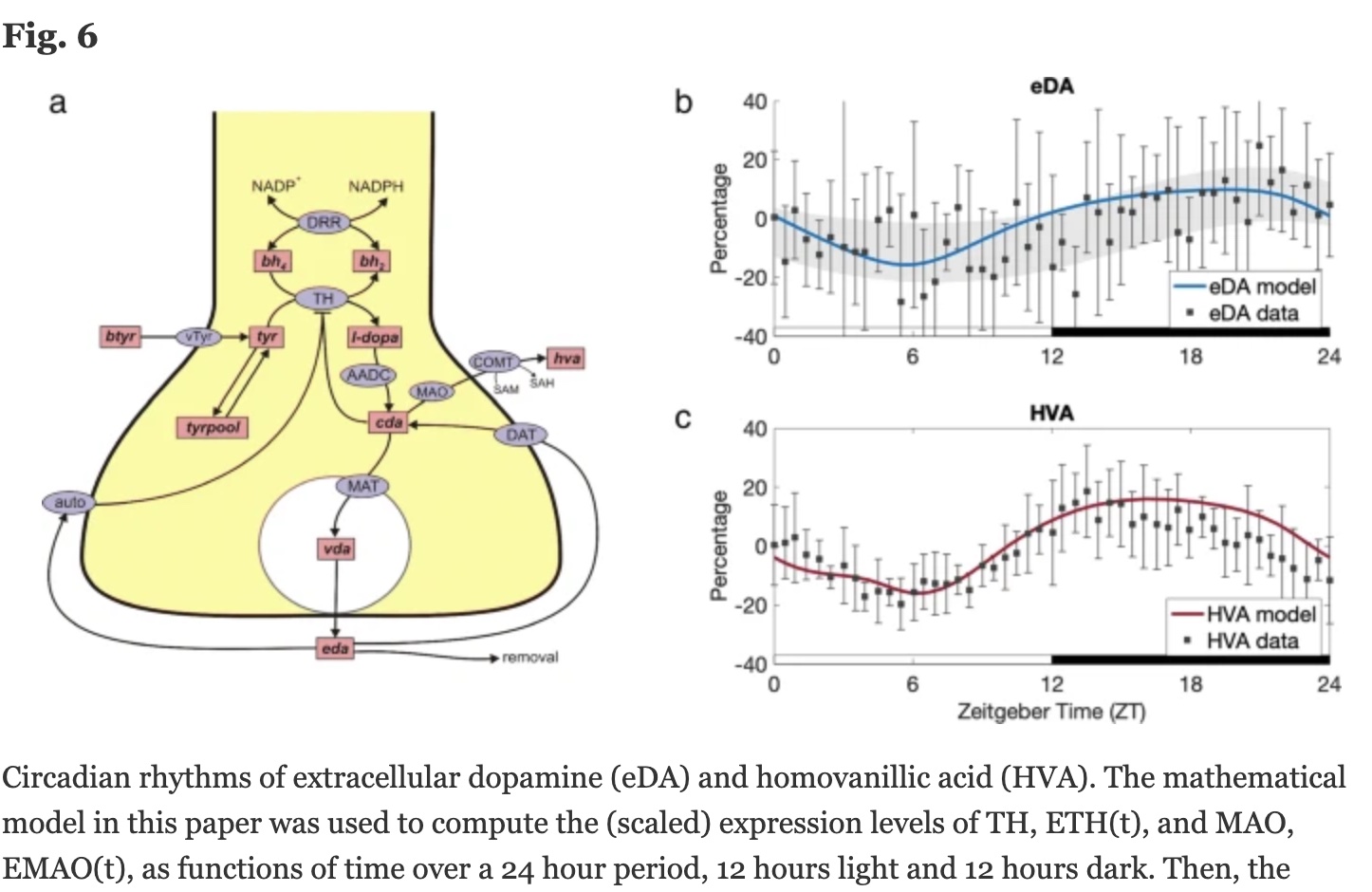
In ADHD, dopamine is often dysregulated. Certain regions of the brain may have higher levels, while other regions may have lower levels. For example, PET scans show that striatal dopamine transporter density is higher in ADHD. Other studies show decreased availability of dopamine receptors in certain brain regions.[ref]
Dysregulation of dopamine levels is at the core of neurodegeneration in Parkinson’s disease. While the symptoms of Parkinson’s are caused by low dopamine due to damaged dopaminergic neurons, the bigger picture shows that excess dopamine may be what drives the damage to the dopaminergic neurons in the first place.
While research is still ongoing, one theory is that excess dopamine due to too much TH damages the dopaminergic neurons, ultimately leading to Parkinson’s disease. Animal models show that overexpression of TH can cause the accumulation of DOPAL ( 3,4-dihydroxyphenylacetaldehyde or DHPA), a toxic metabolite of dopamine, in the brain.[ref][ref]
Important to know with excess dopamine is that aldehyde dehydrogenase (ALDH2) is essential for the conversion of DOPAL (DHPA) into homovanillic acid for excretion. Inhibitors of ALDH2, including certain pesticides, could be one reason for the destruction of the dopaminergic neurons in Parkinson’s.[ref][ref] Aldehyde dehydrogenase is also essential for breaking down the aldehyde formed when alcohol is metabolized.
Excess dopamine in certain brain regions is thought to be the cause of psychosis. It is thought that dopamine excess is part of schizophrenia, although research shows that it isn’t as simple as ‘too much dopamine’. Instead, elevated presynaptic dopamine in the striatum is one finding on PET scans. This is coupled with increased D2 receptors, as shown on SPECT scans.[ref][ref]
Outside of the brain:
While we often think of dopamine just in terms of brain functions, such as reward or feeling good, dopamine is found in the rest of the body at lower levels.
For example, dopamine is synthesized in both the heart and the pancreas. Research also shows that l-DOPA can be taken up in the kidneys for dopamine synthesis. [ref]
In the pancreas, dopamine is thought to play a role in insulin secretion. Animal studies show that plasma glucose and insulin are responsive to the tyrosine content of a test meal.[ref]
Keep in mind that while tyrosine hydroxylase is often referred to as rate-limiting for dopamine, it is also rate-limiting for norepinephrine and epinephrine, two more neurotransmitters in the nervous system. High blood pressure is linked to increased sympathetic nervous system activation due to multiple mechanisms. Variants in the TH gene increase the risk of hypertension, and researchers think this is through the effect on norepinephrine and epinephrine levels. Another term for epinephrine is adrenaline.[ref]
Recent research shows that dopamine is also important in modulating the immune response. Some innate immune cells express dopamine receptors and can synthesize dopamine. For example, macrophages are immune cells that can kill pathogens, and they can be either pro-inflammatory (M1) or anti-inflammatory (M2). Activation of dopamine receptors plays a role in the switch from pro- to anti-inflammatory macrophages. Dopamine also can activate dopamine receptors on T cells. Drugs that are dopamine receptor agonists, therefore, may also impact immune response.[ref]
Genotype report: Dopamine Synthesis Genes
Lifehacks: Supplements for optimizing dopamine
Below you will find information on testing, 11 natural supplements, dietary considerations, and lifestyle changes to optimize dopamine levels.
Testing:
Testing your dopamine levels will help you know where you’re at and if changes to diet and lifestyle are making a difference. Dopamine can be measured in the urine and in the blood. Both types of tests can be ordered by your doctor – or you can order them yourself online. Some lab testing companies, such as Ultalab, offer a fractionated catecholamines test that contains dopamine either as a urine or a blood test.
Homovanillic acid is a metabolite of dopamine that can be measured in the urine. You can have this done by your doctor or you can order it yourself online as part of the ZRT Neurotransmitter Urine Profile (dried urine test). Some tests offer a 24-hour urine test to see how dopamine changes over the course of a day.
Here’s my article on ordering lab tests yourself with some links to companies to check out. Sometimes it is cheaper to order it yourself instead of through your doctor, depending on your insurance. However, even if you order the test yourself, this may be one test that you want to get help with understanding the results by working with your doctor.
Symptoms of high or low dopamine:
Too much dopamine in specific regions of the brain is linked to psychosis and schizophrenia. Physical symptoms can include tic disorders or Tourette syndrome when dopamine is dysregulated in specific brain regions or at the wrong time. It usually isn’t as simple as “high dopamine levels” but instead is dopamine in specific pathways being out of sync.[ref]
Too little dopamine is linked to being less motivated, sad, and having low libido. Physically, low dopamine can cause Parkinson’s symptoms (spasticity, tremors), problems with moving or speaking more slowly than normal, and even digestive and sleep problems.[ref]
Reducing constant dopamine hits:
We live in a world full of stimuli — from constant phone notifications to 20-second video clips to multitasking being the norm. Constant dopamine hits from likes, notifications, etc. Cell phone, internet, and video game addictions are all real and due to dopamine reinforcement/reward. I have a full article on genetics and smartphone addiction if you want to know more.
The simple solution is to take a break from your devices, but that is easier said than done for many. Turning off phone notifications (Instagram, Facebook, Twitter, etc) is a way to start. There are new phones out that are grayscale and not backlit — like a paper and ink version of a phone.
You could try the new TikTok trend called “silent walking”. It’s a newly discovered way of going on a walk without taking your phone so that you can focus on the sounds of nature and be alone with your thoughts. Seriously. You can’t make this stuff up.
Why reduce the constant dopamine hits? Any type of stimulus that causes constant dopamine hits has the potential for addiction. The release of dopamine in the nucleus accumbens drives reward and learning in both good ways and in addiction. The brain is plastic and can be rewired to motivate you to consume more (more time on social media, more sugary snacks, more cocaine). Adaptation to the higher dopamine levels occurs and there is a decrease in the inhibition by GABA and changes to the amygdala. A negative emotional state then ensues when you are deprived of the dopamine hits.[ref]
Again, balance is key.
Supplement stacks for supporting dopamine synthesis:
The following natural vitamins and supplements have research backing them for improving low dopamine levels.
There isn’t a one-size-fits-all supplement for dopamine that will work for everyone (no matter what supplement marketers tell you). Genetics, diet, and lifestyle factors come into play as to whether or not you are likely to benefit from any of these. Use this information to select which supplements will likely be beneficial for you.
Related topics and articles:
Dopamine Receptor SNPs: Addiction, Mood, ADHD, and Schizophrenia
Phenylalanine and Phenylketonuria: Mutations, Carrier Impact
References:
- Peralta, Victor, and Manuel J. Cuesta. “Exploring the Boundaries of the Schizophrenia Spectrum: A Meta-Analysis of the Diagnostic Validity of Schizoaffective Disorder.” Schizophrenia Bulletin, vol. 47, no. 2, 2021, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8543667/.
- Harris, M. G., et al. “Long-Term Outcomes of Psychosis and Suicide Risk: A 10-Year Follow-Up Study.” Journal of Clinical Psychiatry, vol. 72, no. 8, 2011, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3065393/.
- Dubey, Varun, et al. “Genetic Contributions to Schizophrenia and Bipolar Disorder: Insights from Molecular Studies.” Neuropharmacology, vol. 200, 2022, https://www.sciencedirect.com/science/article/pii/S0028390822001174?via%3Dihub.
- Perlis, Roy H. “Clinical Treatment of Bipolar Disorder in Primary Care Settings.” American Journal of Psychiatry, vol. 176, no. 4, 2019, https://pubmed.ncbi.nlm.nih.gov/30714137/.
- Hou, Changzheng, et al. “Genetic Basis of the Association between Epilepsy and Schizophrenia.” Journal of Psychiatric Research, vol. 98, 2018, https://pubmed.ncbi.nlm.nih.gov/29843233/.
- Bipeta, Rajshekhar. “Fronto-Temporal Structural and Functional Differences between Major Depressive Disorder and Bipolar Disorder: An Imaging Study.” Journal of Affective Disorders, vol. 278, 2020, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7702004/.
- Szatmari, Peter, et al. “Comparative Efficacy and Tolerability of Pharmacological Treatments for Acute Mania: A Network Meta-Analysis.” Lancet Psychiatry, vol. 3, no. 9, 2016, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4946061/.
- Liu, Haijun, et al. “Brain Structural Alterations and Cognitive Impairments in Psychotic Major Depression: A Combined Meta-Analysis and VBM Study.” Journal of Affective Disorders, vol. 278, 2021, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8003434/.
- Qiao, Yuncheng, et al. “Epigenetic Regulation of Neurodevelopmental Genes and the Risk of Schizophrenia and Bipolar Disorder.” Journal of Psychiatric Research, vol. 104, 2018, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6215755/.
- Kochunov, Peter, et al. “White Matter Abnormalities in Schizophrenia: A Combined Meta-Analysis of Voxel-Based Morphometry and Diffusion Tensor Imaging Studies.” Neuroimage: Clinical, vol. 14, 2017, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5376559/.
- Goschke, Thomas, and Jens Bölte. “Dynamics of Neuronal Network Activity in Psychotic States: Implications for Diagnosis and Treatment.” Theoretical Biology and Medical Modelling, vol. 18, no. 1, 2021, https://tbiomed.biomedcentral.com/articles/10.1186/s12976-021-00139-w.
- Paulus, Martin P., and Angela R. Laird. “Investigating the Neural Correlates of Psychosis in Schizophrenia: A Meta-Analysis of Task-Based fMRI Studies.” NeuroImage, vol. 213, 2020, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6778824/.
- Mathalon, Daniel H., et al. “Functional Connectivity Abnormalities in Schizophrenia: A Network Meta-Analysis.” Biological Psychiatry, vol. 88, no. 3, 2020, https://pubmed.ncbi.nlm.nih.gov/25068705/.
- Xu, Lei, et al. “Brain Imaging Studies of First-Episode Psychosis: A Systematic Review.” Schizophrenia Bulletin, vol. 46, no. 1, 2020, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6947924/.
- Miller, Paul, et al. “Altered Functional Connectivity in Schizophrenia: A Combined Resting-State and Task-Based fMRI Study.” Neuroimage: Clinical, vol. 14, 2021, https://pubmed.ncbi.nlm.nih.gov/34184261/.
- Seidman, Larry J., et al. “Cognitive and Neuroimaging Predictors of Functional Outcome in Schizophrenia: A Meta-Analysis.” Schizophrenia Research, vol. 224, 2022, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8376767/.
- Addington, Jean, et al. “Functional Connectivity in Individuals at Clinical High Risk for Psychosis: A Meta-Analysis of fMRI Studies.” Schizophrenia Research, vol. 148, no. 1-3, 2013, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3545765/.
- Lencz, Todd, et al. “Biomarkers for the Prediction of Schizophrenia: An fMRI Study.” American Journal of Psychiatry, vol. 171, no. 2, 2014, https://pubmed.ncbi.nlm.nih.gov/24491970/.
- Roth, Bruce L., et al. “Molecular Mechanisms Underlying the Actions of Atypical Antipsychotic Drugs.” Proceedings of the National Academy of Sciences, vol. 91, no. 24, 1994, https://www.pnas.org/doi/abs/10.1073/pnas.91.24.11651.
- Nuechterlein, Keith H., et al. “The MATRICS Consensus Cognitive Battery, Part 1: Test Selection, Reliability, and Validity.” Schizophrenia Bulletin, vol. 35, no. 3, 2009, https://academic.oup.com/schizophreniabulletin/article/35/3/549/1872560.
- Abé, C., et al. “Magnetic Resonance Imaging Predictors of Functional Outcome in Schizophrenia: A Meta-Analysis.” Neuropsychopharmacology, vol. 45, no. 5, 2022, https://pubmed.ncbi.nlm.nih.gov/33737022/.
- Kubota, Maki, et al. “Impaired Neuronal Maturation and Cognitive Deficits in Schizophrenia: A Meta-Analysis of VBM Studies.” NeuroImage: Clinical, vol. 18, 2019, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6479665/.
- Cullen, Katherine R., et al. “Cognitive and Structural Brain Changes in Schizophrenia: A Longitudinal MRI Study.” Biological Psychiatry, vol. 81, no. 6, 2017, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5337429/.
- Fusar-Poli, Paolo, et al. “Neurobiological Predictors of Psychosis in the At-Risk Mental State: A Meta-Analysis of VBM Studies.” Comptes Rendus Biologies, vol. 345, no. 12, 2021, https://www.sciencedirect.com/science/article/pii/S0753332221012440?via%3Dihub.