Key takeaways:
The serotonin 2A receptor is important in regulating memory, mood, cognition, appetite, anxiety, perception, sleep, thermoregulation, and vasoconstriction.
~ This is also the receptor that psychedelics act upon, and this may be important in Alzheimer’s disease.
~ Variants in the serotonin 2A receptor influence the risk of Alzheimer’s disease as well as response to medications.
This article digs into current research on the serotonin 2A receptor, psychedelics response, and genetic variants — with a focus on brain aging and dementia.
Serotonin and the Serotonin 2A Receptor
What do Alzheimer’s disease and LSD have in common? The serotonin 2A receptor…
Clinical trials are underway for using low-dose LSD or psilocybin, two psychedelic drugs that bind to the serotonin 2A receptor, to treat Alzheimer’s disease.
Serotonin is a neurotransmitter created from the amino acid tryptophan. Most of the body’s serotonin is found in the intestines, where it acts as a neurotransmitter controlling intestinal peristalsis.
In the brain, serotonin helps to transmit signals between neurons. It is involved in learning, mood, memory, and emotions. Only about 1 – 2% of the body’s serotonin is found in the brain.[ref]
Serotonin cannot cross the blood-brain barrier, so it has to be synthesized in the brain from tryptophan. The body tightly regulates serotonin levels, and it is broken down in the brain by the MAO (monoamine oxidase) enzyme. MAO inhibitors are a class of anti-depressant medications.
Recent research also elucidates the role of serotonin in immune function.[ref] This ties in with the role of serotonin in mood disorders as well as the role of inflammation in aging and dementia. (Read more about inflammation, genetics, anxiety, and depression.)
Serotonin Receptors
For serotonin to send a signal between neurons, the end of one neuron releases it to bind to a serotonin receptor of the next neuron. Activating the receptor then causes an action to occur in the next neuron.
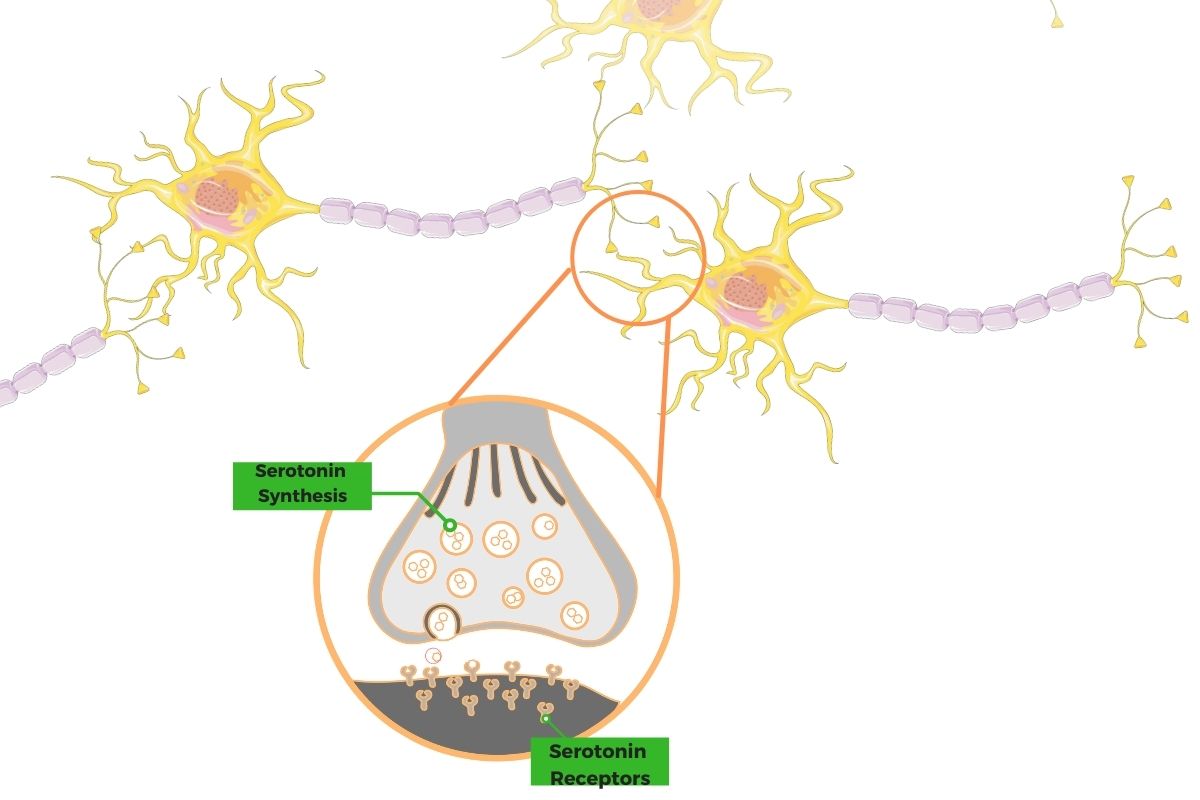
Serotonin is present in all animals and acts as either a hormone or a neurotransmitter.
There are seven different families of receptors for serotonin in the human body, causing different actions to occur via binding to serotonin. The receptors are known as 5-HT or 5-hydroxytryptamine receptors.
The serotonin 2A receptor (5-HT2A) is found throughout the body, including in the brain. It is important in regulating memory, mood, cognition, appetite, anxiety, perception, sleep, thermoregulation, and vasoconstriction.
When activated, the effect of the serotonin 2A receptor is to increase the excitability of the neuron. The activation of this receptor causes:
- In neurons that are excitatory, activation of the serotonin 2A receptor causes the neuron to increase firing.
- In neurons that are inhibitory, such as GABAergic neurons, activation of the serotonin 2A receptor results in inhibiting neighboring neurons.
- Some neurons have more than one type of serotonin receptor with different effects.
In other words, this isn’t a straightforward equation of serotonin 2A = excitation in the brain.[ref]
So why do we care about serotonin 2A? It causes some unique effects in the brain, which could be important for mood, cognition, and preventing dementia.
Psychoactive chemicals and serotonin 2A
Humans have been using psychoactive chemicals in various forms throughout history. From Native Americans using peyote (mescaline) to Mazatec priests using psilocybin mushrooms, many historical uses of natural psychedelics have been recorded.
While serotonin is the naturally produced ligand that binds to the serotonin 2A receptor, certain drugs, and natural substances also can bind to this receptor, causing interesting effects.
Drugs that bind to the serotonin 2A receptor include LSD (lysergic diethylamide), mescaline, DMT, and psilocybin.[ref]
Hallucinogens at higher levels cause altered states of consciousness – changes in mood, perception, and thinking. Animal experiments show that binding with the serotonin 2A receptor, also known as the 5-HT2A receptor, is necessary for the hallucinogenic effects of certain drugs.
Interestingly, there are a few drugs that bind to the serotonin 2A receptor that do not cause hallucinations or altered cognitive states. These include lisuride (Parkinson’s medication) and ergotamine (migraine med).[ref] It seems to depend on how strongly different parts of the receptor are activated. Ergotamine is derived from ergot, which is a fungal growth on rye plants that can cause hallucinations.
What else does the serotonin 2A receptor impact?
To determine the function of the receptor, researchers used low doses of LSD as a serotonin 2A receptor agonist (something that binds to and activates the receptor). In comparison, many studies also use a placebo control group as well as a drug that inactivates the serotonin 2A receptor. This allows the investigators to determine the exact effect on the serotonin 2A receptor.
- Social cognition: Another study shows the serotonin 2A receptor’s importance in social cognition and the sense of self in relation to others.[ref]
- Adaptation to a group: A 2020 study shows the serotonin 2A receptor increases “social adaptation but only if the opinions of others are similar to the individual’s own.”[ref]
- Relevance of stimuli: Researchers found that the serotonin 2A receptor alters how people find meaning from stimuli.[ref]
- Active coping: Researchers theorize that the serotonin 2A receptor is important for brain plasticity and the ability to actively cope with a stressor.[ref]
- Music: A recent study investigated the role of the serotonin 2A receptor in people who were listening to music. The researchers found that the serotonin 2A receptor is critical in a person’s response to music, such as the emotions, connections, and meaningfulness of the music.[ref]
The laws on testing psychedelics have just been changed in the past couple of years, allowing research on their efficacy in treating mood disorders. Prior to this, laws put into place in the 1970s during the ‘war on drugs’ in the US kept these compounds from being available for research.
The Aging Brain: why psychedelics and the serotonin 2A receptor may be important
Studies over the last decade have investigated the effects of serotonin 2A receptor agonists on depression and anxiety. Specifically, researchers have found:
- A high, single dose of psilocybin produced a large decrease in depression and anxiety in cancer patients[ref]
- Various doses of LSD, along with psychotherapy, decrease anxiety in people with life-threatening illnesses[ref]
- In a small trial, a single dose of ayahuasca alleviated depression in patients with recurrent depression[ref]
As we age, the number of serotonin 2A receptors in the brain decreases significantly.[ref]
Importantly, these receptors are located in parts of the brain impacted by dementia, including the prefrontal cortex and the hippocampus.[ref]
The plasticity of the brain is the ability of the brain to change and grow.
Psilocybin and LSD increase neurogenesis – the growth of neurons – and induce brain plasticity.[ref]
Importantly, psychedelics can increase brain plasticity at very low doses (microdoses), which do not impair function or cause hallucinations.[ref]
Additionally, safety studies on microdoses of LSD and ayahuasca showed no detrimental side effects.[ref][ref] Most of the studies are rather small, though, so larger and more rigorous studies are needed here.
Alzheimer’s and serotonin 2A receptors:
Alzheimer’s disease remains the primary cause of dementia and affects about 10% of people over the age of 65. This creates a huge economic burden, as well as greatly impacts family members who act as caregivers. While mortality rates for stroke, heart disease, and certain cancers are decreasing, the number of deaths for Alzheimer’s increased by 71% between 2000 and 2013.[ref] Current numbers are even worse.
In Alzheimer’s patients, the expression of the serotonin 2A receptors is significantly decreased in the brain when compared to people their age without cognitive dysfunction. One study estimated a 33% decrease in receptor expression, which is a large change compared to age-matched controls. Other studies show the reduction in serotonin 2A receptors occurs early in Alzheimer’s when cognitive dysfunction is mild.[ref][ref][ref][ref][ref]
Research shows reduced serotonin levels in the cortex of people who had psychosis with Alzheimer’s disease (postmortem study).
The study also shows a genetic variant in the serotonin 2A receptor gene has associations with an increased risk of psychosis in Alzheimer’s disease. Psychosis in Alzheimer’s may not be entirely genetic, though. In addition to the serotonin 2A receptor variants, it is likely that psychosis in Alzheimer’s also involves environmental factors such as medication side effects, angry caregivers, or stressful changes.[ref][ref]
Additionally, MAO-A levels are also decreased in Alzheimer’s. MAO-A (monoamine oxidase) is the enzyme that breaks down serotonin, regulating the amount of serotonin available.[ref]
I mentioned above that serotonin also impacts the immune system, which is integral to the formation of amyloid-beta plaque in the brain (increased in Alzheimer’s patients). Along with elevated cytokines such as TNF-alpha, the reduction of serotonin has been shown to impact the formation of amyloid-beta plaques as well as increase depression in Alzheimer’s patients.[ref][ref][ref]
Will increasing serotonin help reverse Alzheimer’s? Probably not.
While animal studies on SSRIs were initially exciting, SSRI studies for Alzheimer’s patients showed mixed results. SSRIs increase the amount of time that serotonin hangs out in the synapses of the neurons, but it doesn’t seem to reverse or stop Alzheimer’s pathology in humans, at least in the short term.[ref][ref][ref][ref]
On the other hand, long-term prior use of SSRIs for depression may delay the progression of Alzheimer’s dementia.[ref][ref] While most of the antidepressant effects of SSRIs are thought to come from the serotonin 1A receptor, recent genetic studies point to the role of the serotonin 2A receptor in the long-term effects of SSRIs.[ref]
So why would a serotonin 2A agonist, such as LSD, reduce Alzheimer’s pathology? Increasing the amount of time that serotonin hangs out in the synapse in the brain doesn’t necessarily increase the serotonin 2A receptors in the aging brain.
On the other hand, psychedelic drugs increase the outgrowths of the serotonergic neurons, thus increasing the number of serotonin 2A receptors.[ref]
Increasing neurogenesis, plasticity, and brain connectedness – rather than a focus on serotonin – seems to be the key here.[ref][ref]
Activating the serotonin 2A receptor also increases mitochondrial biogenesis (formation of new mitochondria).[ref] Alzheimer’s disease is also linked to decreased mitochondrial function in the brain.
Repeated microdoses of LSD are in clinical trials in older adults to determine safety and efficacy for Alzheimer’s prevention. Initial phase I clinical trial results show that low doses of LSD are well tolerated without adverse events.[ref]
HTR2A Genotype Report:
If you want to know more about your genetic risk of Alzheimer’s disease, you can check your APOE type.
Lifehacks:
Please note that I’m not advocating that you microdose LSD, psilocybin, or do anything illegal. The information on the research studies is presented for informational purposes, and it truly is preliminary.
A lot more research needs to be done on this topic before psychedelics can be used for Alzheimer’s, but it is exciting to see this avenue of research being pursued. For example, John’s Hopkins University is currently conducting clinical trials using psilocybin in Alzheimer’s patients.[ref]
So what can you do to alter serotonin and the serotonin 2A receptors in the brain if you live in a place where psychedelics are illegal?
Related Articles and Topics:
MAO-A and MAO-B: Neurotransmitter levels, genetics, and warrior gene studies