Key takeaways:
~ Low dose naltrexone is effective for some people with autoimmune diseases, pain syndromes, and chronic fatigue syndrome.
~ LDN modulates the immune response in a couple of different ways, including blocking TLR4 activation and impacting ion channels on natural killer cells.
~ Genetics plays a role in response to naltrexone by the mu-opioid receptor, and genetic polymorphisms also impact the metabolism of naltrexone.
<b>Members</b> will see their genotype report below and the solutions in the Lifehacks section. <a href=”https://www.geneticlifehacks.com/membership/”>Consider joining today</a>.
Low Dose Naltrexone:
Low dose naltrexone (LDN) is an off-label use of the opioid antagonist naltrexone.
Naltrexone is a competitive antagonist at the μ-opioid receptor (MOR). This means that it binds to the opioid receptor, blocks other opioids from the receptor, but also doesn’t activate the receptor. Naltrexone in higher doses (e.g., 50mg) treats alcohol and opioid drug addiction.
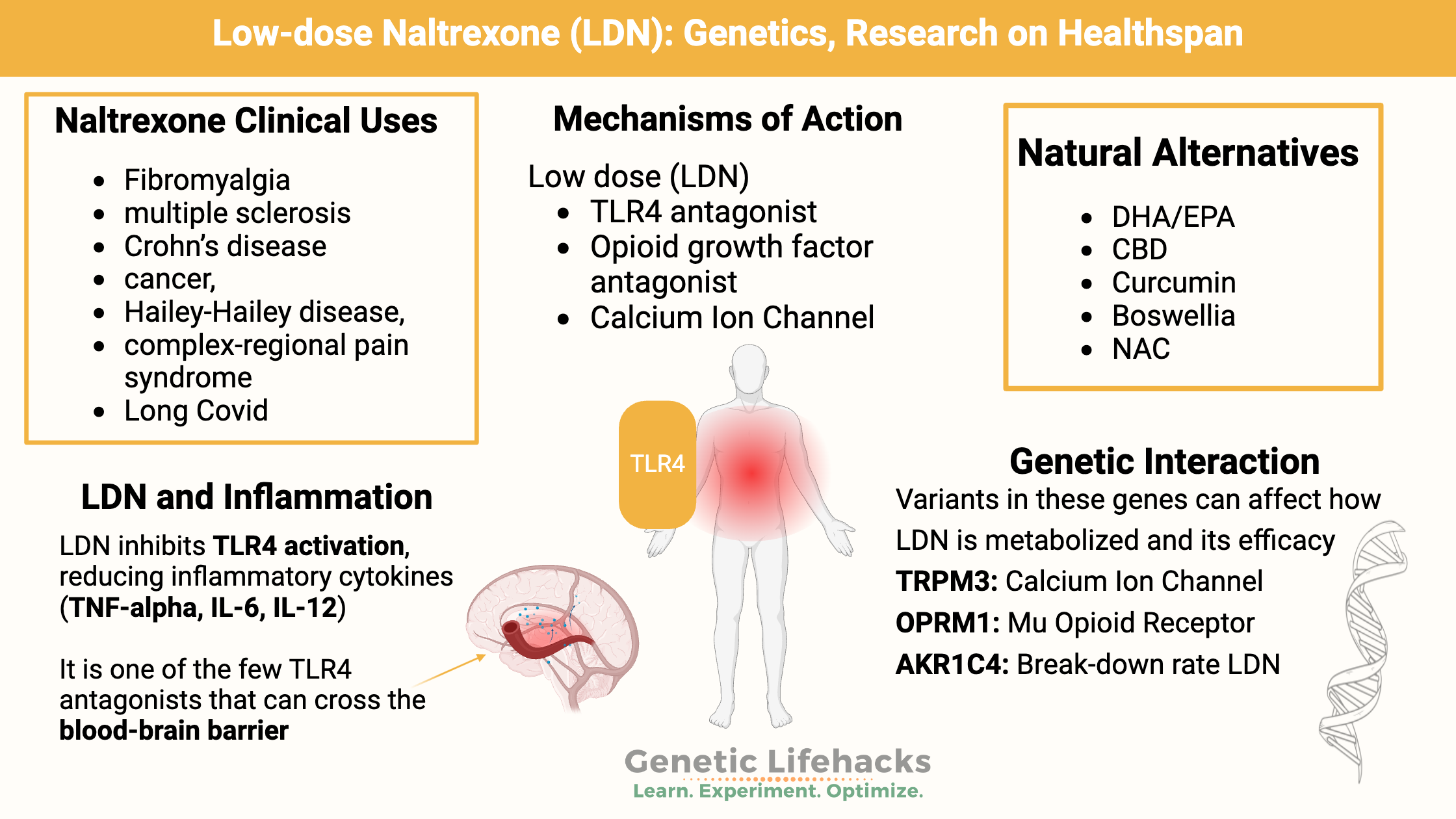
Low dose naltrexone is now used for several types of autoimmune diseases (MS, Crohn’s disease) and pain syndromes such as fibromyalgia.
Initially, in the 1980s, low dose naltrexone was prescribed in the 1.5-3mg range for AIDS patients. Currently, LDN doses can be 1-5mg or even lower – in the 1 mcg range.[ref]
LDN works in a different way compared to higher doses of naltrexone. The levels at which higher doses block the opioid receptor are different than the LDN levels, which act on different receptors.
(Adapted from PMC6313374)
| Dose Range | Dose Specific Mechanism of Action | Clinical Use |
|---|---|---|
| Standard (50–100 mg) |
Opioid receptor antagonism | Alcohol and opioid drug addiction |
| Low-dose naltrexone (1–5 mg) |
Toll-like receptor 4 (TLR4) antagonism, opioid growth factor antagonism | Fibromyalgia, multiple sclerosis, Crohn’s disease, cancer, Hailey-Hailey disease, complex-regional pain syndrome |
| Very low-dose (1mcg–1 mg) | Possibly same as low dose | Add-on to methadone detoxification taper |
How does LDN impact Inflammation?
One way that LDN is thought to work is by inhibiting TLR4 (toll-like receptor 4). It is also thought to inhibit the proliferation of T and B cells.[ref]
TLR4 is a receptor that recognizes lipopolysaccharide (LPS) on the surface of gram-negative bacteria. In addition, TLR4 binds to certain molecules produced as a result of tissue injury. TLR4 is expressed on the cell surface of endothelial cells (lining of blood vessels), cardiac myocytes (heart muscle), and cells in the central nervous system. When TLR4 is activated by bacterial LPS or by molecules from injured cells, it triggers a cascade of events that causes the cell to produce inflammatory cytokines.[ref]
LDN is thought to block the activation of TLR4, thus reducing the release of inflammatory cytokines such as TNF-alpha, IL-6, and IL-12.[ref]
LDN is one of the few TLR4 antagonists that can cross the blood-brain barrier.[ref] An animal study of multiple sclerosis found that naltrexone prevents the learning and memory issues associated with neuroinflammation. This study also found that in rats, naltrexone also blocks TLR2 activation in addition to blocking TLR4 activation.[ref]
Mechanism of action of LDN in ME/CFS:
A recent study looked at one way that low dose naltrexone may work for people with chronic fatigue syndrome. Calcium ions are essential in intracellular signaling, including for immune cells such as natural killer cells.
A 2016 study identified variants in the TRPM3 gene as impacting the relative risk of ME/CFS. The TRPM3 gene encodes a calcium channel on natural killer (NK) cells, and in people with ME/CFS, there was a loss of the TRPM3 channel in NK cells. One hypothesis now is that ME/CFS is caused, or partly caused, by impaired TRPM3 ion channels.[ref]
Naltrexone acts on the mu opioid receptors. Researchers have found that TRPM3 channels are strongly inhibited by peripheral mu opioid receptor activation.[ref] This may be the connection as to why LDN helps some people with ME/CFS.[ref –open access, worth reading]
LDN for MS:
Clinical trials of LDN in relapsing-remitting multiple sclerosis show conflicting results.
- One placebo-controlled trial of 4.5 mg LDN nightly showed statistically significant improvements in mental health and pain scales.[ref]
- However, another placebo-controlled trial, though, showed no statistical difference between LDN and placebo in MS patients.[ref]
In a review of six trials of LDN in MS, four out of six had positive results.[ref]
LDN for pain:
At low doses, naltrexone has a pain-relieving effect. (At high doses, naltrexone blocks the opioid receptor and doesn’t stop pain.)
Several clinical trials have evaluated low dose naltrexone for pain management in complex regional pain syndrome and fibromyalgia. Many trials showed positive results. For example, a study using 4.5 mg LDN for fibromyalgia showed a 30% reduction in symptoms compared to baseline.[ref]
LDN for chronic fatigue syndrome (ME/CFS):
A number of case studies of LDN for ME/CFS show that it can bring recovery to some people. The case studies show that finding the right dose takes time and experimentation. The optimal dose and schedule was different for everyone. The cases presented all started with very low doses, and they worked with a doctor to slowly increase the dose and then choose the right amount.[ref]
LDN and Long Covid:
An initial safety study in Long Covid patients showed that LDN improved energy levels, sleep, concentration, and daily living.[ref]
LDN interacts with and blocks TLR-4, thereby modulating the inflammatory response. Thus, researchers have theorized it may help with COVID-19 and Long Covid symptoms.[ref] This may be especially true for persistent, low-level SARS-CoV-2 infections.
A recent study also showed that the same TRMP3 ion channel impairment was also found in patients with Long Covid. Similar to ME/CFS patients, the TRPM3 dysfunction in natural killer cells also existed in Long Covid patients but not in healthy controls.[ref]
Safety and Side Effects:
Full doses of naltrexone (50-100mg) are considered safe. However, some report behavioral changes as side effects. There is no increased risk of serious adverse events with naltrexone compared with a placebo, according to a Cochrane analysis.[ref]
Clinical trials of LDN also show that it is safe, but side effects such as vivid dreams and insomnia have been noted when people start taking LDN at bedtime.[ref]
Of course, talk to your doctor before starting LDN or any medication. There may be individual cases where it is not well tolerated.
Naltrexone metabolism:
The rate at which a drug is broken down (metabolized) affects how long it stays in your system.
Naltrexone is primarily metabolized by AKR1C4 (ald0-keto reductase 1C4). Genetic variants in AKR1C4 can cause a 5-fold reduction in enzyme activity. Recent research shows that these variants affect the metabolism of naltrexone.[ref]
In addition, AKR1C4 is also involved in the biotransformation of testosterone. At certain levels, testosterone has been shown to inhibit the metabolism of naltrexone.[ref] This may be something to keep in mind for women or men on testosterone therapy, as well as a difference in naltrexone dosing between men and women.
Naltrexone Genotype Report:
Members: Log in to see your data below.
Not a member? Join here.
Why is this section is now only for members? Here’s why…
Lifehacks: LDN metabolism, sources, and natural alternatives
The rest of this article is for Genetic Lifehacks members only. Consider joining today to see the rest of this article.
Related Articles and Topics
Opioid Receptors: Variants and addiction
Genetic variants in the ORPM1 gene impact both the amount of pain someone experiences and their response to opioid drugs. These variants are also important in susceptibility to opiate addiction.
Decoding the Genetic Factors Behind Multiple Sclerosis
MS susceptibility is linked to both genetic causes and environmental factors. Learn how those two combine – and possible solutions.
HLA B27: Genetic Variant That Increases Susceptibility to Autoimmune Diseases
Our immune system does an awesome job (most of the time) of fighting off pathogenic bacteria and viruses. But to fight off these pathogens, the body needs to know that they are the bad guys. This is where the HLA system comes in.
References:
Anton, Raymond F., Gabor Oroszi, et al. “An Evaluation of Mu-Opioid Receptor (OPRM1) as a Predictor of Naltrexone Response in the Treatment of Alcohol Dependence: Results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) Study.” Archives of General Psychiatry, vol. 65, no. 2, Feb. 2008, pp. 135–44. PubMed, https://doi.org/10.1001/archpsyc.65.2.135.
Anton, Raymond F., Konstantin E. Voronin, et al. “Opioid and Dopamine Genes Interact to Predict Naltrexone Response in a Randomized Alcohol Use Disorder Clinical Trial.” Alcoholism, Clinical and Experimental Research, vol. 44, no. 10, Oct. 2020, pp. 2084–96. PubMed Central, https://doi.org/10.1111/acer.14431.
Aslaksen, Per M., et al. “The Opioid Receptor Mu 1 (OPRM1) Rs1799971 and Catechol-O-Methyltransferase (COMT) Rs4680 as Genetic Markers for Placebo Analgesia.” Pain, vol. 159, no. 12, Dec. 2018, pp. 2585–92. PubMed, https://doi.org/10.1097/j.pain.0000000000001370.
Bolton, Monica, et al. “Serious Adverse Events Reported in Placebo Randomised Controlled Trials of Oral Naltrexone: A Systematic Review and Meta-Analysis.” BMC Medicine, vol. 17, no. 1, Jan. 2019, p. 10. PubMed, https://doi.org/10.1186/s12916-018-1242-0.
Bolton, Monica Jane, et al. “Low-Dose Naltrexone as a Treatment for Chronic Fatigue Syndrome.” BMJ Case Reports, vol. 13, no. 1, Jan. 2020, p. e232502. PubMed Central, https://doi.org/10.1136/bcr-2019-232502.
Borsa, Paul A., et al. “Genetic and Psychological Factors Interact to Predict Physical Impairment Phenotypes Following Exercise-Induced Shoulder Injury.” Journal of Pain Research, vol. 11, 2018, pp. 2497–508. PubMed, https://doi.org/10.2147/JPR.S171498.
Britch, Stevie C., et al. “Cannabidiol: Pharmacology and Therapeutic Targets.” Psychopharmacology, vol. 238, no. 1, Jan. 2021, pp. 9–28. PubMed Central, https://doi.org/10.1007/s00213-020-05712-8.
Butler, Michael J., et al. “Dietary DHA Prevents Cognitive Impairment and Inflammatory Gene Expression in Aged Male Rats Fed a Diet Enriched with Refined Carbohydrates.” Brain, Behavior, and Immunity, vol. 98, Nov. 2021, pp. 198–209. PubMed, https://doi.org/10.1016/j.bbi.2021.08.214.
Cabanas, H., et al. “Validation of Impaired Transient Receptor Potential Melastatin 3 Ion Channel Activity in Natural Killer Cells from Chronic Fatigue Syndrome/ Myalgic Encephalomyelitis Patients.” Molecular Medicine (Cambridge, Mass.), vol. 25, no. 1, Apr. 2019, p. 14. PubMed, https://doi.org/10.1186/s10020-019-0083-4.
Cree, Bruce A. C., et al. “Pilot Trial of Low-Dose Naltrexone and Quality of Life in Multiple Sclerosis.” Annals of Neurology, vol. 68, no. 2, Aug. 2010, pp. 145–50. PubMed, https://doi.org/10.1002/ana.22006.
Daily, James W., et al. “Efficacy of Turmeric Extracts and Curcumin for Alleviating the Symptoms of Joint Arthritis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials.” Journal of Medicinal Food, vol. 19, no. 8, Aug. 2016, pp. 717–29. PubMed Central, https://doi.org/10.1089/jmf.2016.3705.
Dembla, Sandeep, et al. “Anti-Nociceptive Action of Peripheral Mu-Opioid Receptors by G-Beta-Gamma Protein-Mediated Inhibition of TRPM3 Channels.” ELife, vol. 6, 2017. www.ncbi.nlm.nih.gov, https://doi.org/10.7554/eLife.26280.
Eaton-Fitch, Natalie, et al. “Impaired TRPM3-Dependent Calcium Influx and Restoration Using Naltrexone in Natural Killer Cells of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients.” Journal of Translational Medicine, vol. 20, Feb. 2022, p. 94. PubMed Central, https://doi.org/10.1186/s12967-022-03297-8.
Hwang, In Cheol, et al. “OPRM1 A118G Gene Variant and Postoperative Opioid Requirement: A Systematic Review and Meta-Analysis.” Anesthesiology, vol. 121, no. 4, Oct. 2014, pp. 825–34. PubMed, https://doi.org/10.1097/ALN.0000000000000405.
Kwilasz, Andrew J., et al. “Experimental Autoimmune Encephalopathy (EAE)-Induced Hippocampal Neuroinflammation and Memory Deficits Are Prevented with the Non-Opioid TLR2/TLR4 Antagonist (+)-Naltrexone.” Behavioural Brain Research, vol. 396, Jan. 2021, p. 112896. PubMed, https://doi.org/10.1016/j.bbr.2020.112896.
Marshall-Gradisnik, Sonya, et al. “Natural Killer Cells and Single Nucleotide Polymorphisms of Specific Ion Channels and Receptor Genes in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome.” The Application of Clinical Genetics, vol. 9, Mar. 2016, pp. 39–47. PubMed Central, https://doi.org/10.2147/TACG.S99405.
Molteni, Monica, et al. “The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation.” Mediators of Inflammation, vol. 2016, 2016, p. 6978936. PubMed Central, https://doi.org/10.1155/2016/6978936.
O’Kelly, Brendan, et al. “Safety and Efficacy of Low Dose Naltrexone in a Long Covid Cohort; an Interventional Pre-Post Study.” Brain, Behavior, & Immunity – Health, vol. 24, July 2022, p. 100485. PubMed Central, https://doi.org/10.1016/j.bbih.2022.100485.
Patten, Denise K., et al. “The Safety and Efficacy of Low-Dose Naltrexone in the Management of Chronic Pain and Inflammation in Multiple Sclerosis, Fibromyalgia, Crohn’s Disease, and Other Chronic Pain Disorders.” Pharmacotherapy, vol. 38, no. 3, Mar. 2018, pp. 382–89. PubMed, https://doi.org/10.1002/phar.2086.
Pei, Yanping, et al. “Biological Activities and Potential Oral Applications of N-Acetylcysteine: Progress and Prospects.” Oxidative Medicine and Cellular Longevity, vol. 2018, 2018. www.ncbi.nlm.nih.gov, https://doi.org/10.1155/2018/2835787.
Porter, Susan J., et al. “Kinetics and Inhibition of the Formation of 6β-Naltrexol from Naltrexone in Human Liver Cytosol.” British Journal of Clinical Pharmacology, vol. 50, no. 5, Nov. 2000, pp. 465–71. PubMed Central, https://doi.org/10.1046/j.1365-2125.2000.00281.x.
Raghu, Ganesh, et al. “The Multifaceted Therapeutic Role of N-Acetylcysteine (NAC) in Disorders Characterized by Oxidative Stress.” Current Neuropharmacology, vol. 19, no. 8, Aug. 2021, pp. 1202–24. PubMed Central, https://doi.org/10.2174/1570159X19666201230144109.
Ray, Lara A., et al. “Pharmacogenetics of Naltrexone in Asian Americans: A Randomized Placebo-Controlled Laboratory Study.” Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, vol. 37, no. 2, Jan. 2012, pp. 445–55. PubMed, https://doi.org/10.1038/npp.2011.192.
Serhan, Charles N. “Novel Pro-Resolving Lipid Mediators in Inflammation Are Leads for Resolution Physiology.” Nature, vol. 510, no. 7503, June 2014, pp. 92–101. PubMed Central, https://doi.org/10.1038/nature13479.
Sharafaddinzadeh, Naser, et al. “The Effect of Low-Dose Naltrexone on Quality of Life of Patients with Multiple Sclerosis: A Randomized Placebo-Controlled Trial.” Multiple Sclerosis (Houndmills, Basingstoke, England), vol. 16, no. 8, Aug. 2010, pp. 964–69. PubMed, https://doi.org/10.1177/1352458510366857.
Stancil, Stephani L., Michaela Voss, et al. “Effects of Genotype and Food on Naltrexone Exposure in Adolescents.” Clinical and Translational Science, vol. 15, no. 11, Nov. 2022, pp. 2732–43. DOI.org (Crossref), https://doi.org/10.1111/cts.13399.
Stancil, Stephani L., Whitney Nolte, et al. “The Impact of Age and Genetics on Naltrexone Biotransformation.” Drug Metabolism and Disposition, vol. 50, no. 2, Feb. 2022, pp. 168–73. PubMed Central, https://doi.org/10.1124/dmd.121.000646.
Toljan, Karlo, and Bruce Vrooman. “Low-Dose Naltrexone (LDN)—Review of Therapeutic Utilization.” Medical Sciences, vol. 6, no. 4, Sept. 2018, p. 82. PubMed Central, https://doi.org/10.3390/medsci6040082.
Vishal, Amar A., et al. “A Double Blind, Randomized, Placebo Controlled Clinical Study Evaluates the Early Efficacy of Aflapin in Subjects with Osteoarthritis of Knee.” International Journal of Medical Sciences, vol. 8, no. 7, 2011, pp. 615–22. PubMed, https://doi.org/10.7150/ijms.8.615.
Zhao, Zhonghai, et al. “Effects of OPRM1 and ABCB1 Gene Polymorphisms on the Analgesic Effect and Dose of Sufentanil after Thoracoscopic-Assisted Radical Resection of Lung Cancer.” Bioscience Reports, vol. 39, no. 1, Jan. 2019, p. BSR20181211. PubMed, https://doi.org/10.1042/BSR20181211.