Key takeaways:
~ Multiple Sclerosis is a disease that causes neuroinflammation and demyelination.
~ Susceptibility to MS is linked to variants in genes related to immune response, inflammation, circadian rhythm, and fatty acids.
~ Understanding the underlying causes may help people with MS find solutions for improving their quality of life.
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Consider joining today.
Genetic Involvement and Root Causes of Multiple Sclerosis (MS)
Multiple Sclerosis (MS) is a disease marked by inflammation and demyelination, primarily affecting the brain and spinal cord within the central nervous system.
The exact causes of MS are not fully understood; however, research shows that a combination of environmental factors and genetic predisposition play a role.
The National Multiple Sclerosis Society explains that MS is: “an unpredictable, often disabling disease of the central nervous system that disrupts the flow of information within the brain and between the brain and body.”
As of 2016, there were over 2 million cases of MS reported worldwide. MS is more common in the US, Canada, Europe, and Australia compared to Africa and Oceania. Let me put this into context: In the US, Europe, and Australia, the prevalence is ~1% of adults. In developing countries, it is less than 0.2%. [ref]
The onset of MS is believed to be linked to immune system alterations and the breakdown of the brain’s protective barrier. These changes lead to the destruction of the myelin sheath around nerve fibers and subsequent loss of nerve fibers, leading to problems with movement, sensations, and cognitive function.[ref]
Developing MS
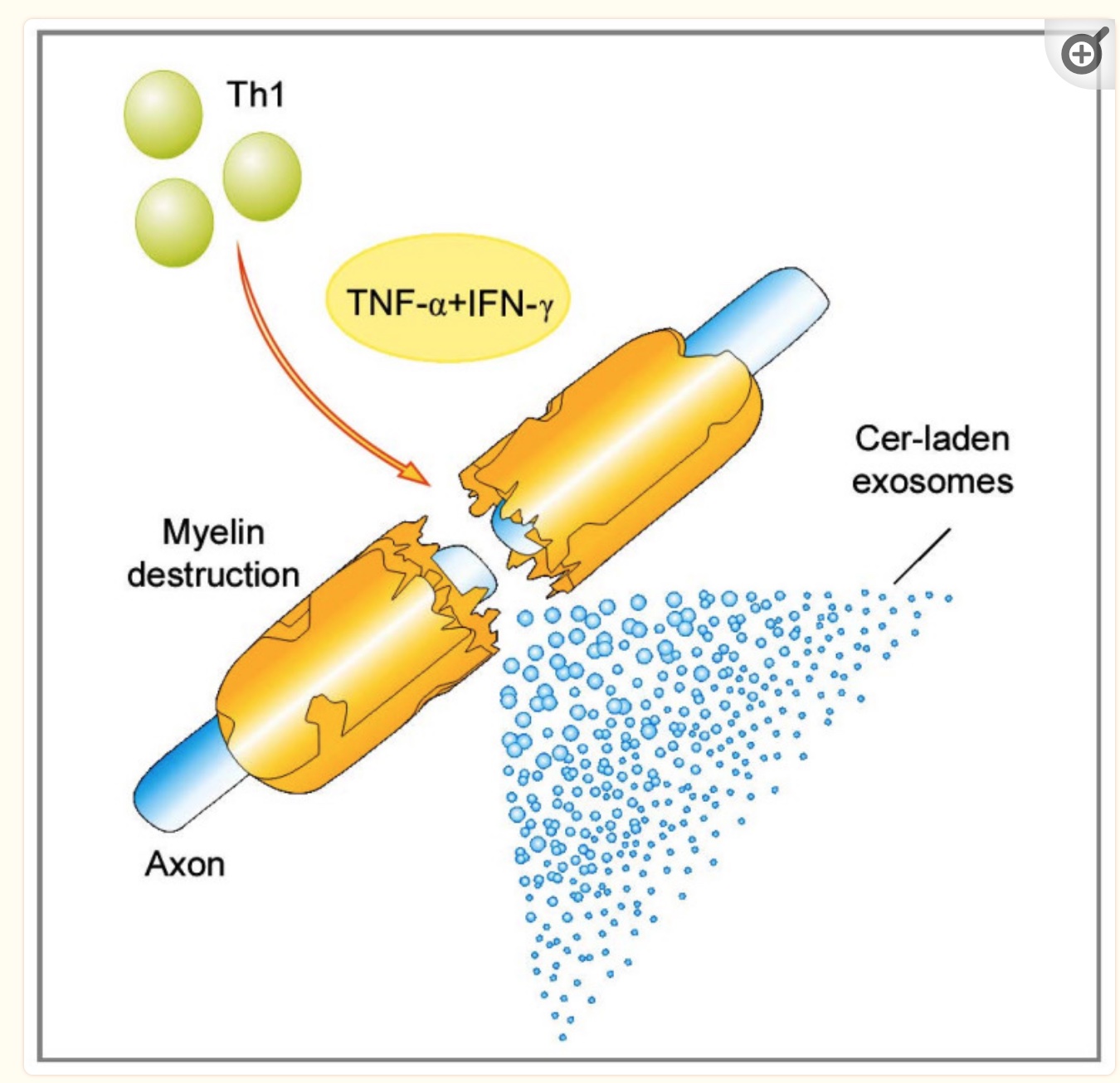
Currently, some researchers think multiple sclerosis is an autoimmune disease with autoreactive Th1 (T helper 1) cells and Th17 cells.
In an autoimmune disease, an antigen causes the immune system to attack healthy cells. In MS, the initial antigen triggering the disease is still not known. It is theorized that contact with an unknown antigen causes excessive production of inflammatory cytokines (IL-1 and interferon-gamma) by the Th1 and Th17 cells. T helper cells (Th1 and Th17) are immune cells that activate B cells and initiate a long-term immune response against a pathogen — or, in this case, against certain cell types.
In MS, the activation snowballs into the upregulation of more T helper cells and, eventually, problems with the blood-brain barrier (BBB). When the BBB is damaged, T helper cells and B cells infiltrate the central nervous system, causing inflammation there.[ref]
New research raises questions on whether MS is indeed an autoimmune disease or a disease of immune system over-activation.[ref]
The distinction here is that traditional autoimmune diseases have specific autoantibodies formed. In MS, only some of the patients have autoantibodies.
How is MS diagnosed?
The main symptoms of MS include:
- Extreme tiredness and fatigue
- Numbness and tingling
- Muscle spasms, stiffness, and pain
- Difficulty walking, dizziness, and sensations of spinning
- Vision problems (optic neuritis) or hearing loss
- Speech and swallowing problems
- Bladder, bowel, or sexual problems
- Cognitive and mental health issues
- Headache
- Seizures
The symptoms of MS alone are not enough to diagnose the condition, though. Plus, there aren’t quick blood tests that can definitively diagnose MS. This is why there can sometimes be ambiguity or misdiagnosis.
Diagnosis of MS usually includes MRI or PET scans showing active inflammatory spots in the central nervous system. Immunoglobulin (IgG) is usually measured. Nervous system and coordination tests may be done, and blood tests can be used to rule out other causes of neurological damage.[ref][ref]
Misdiagnosis is a common (and frustrating!) experience for autoimmune patients. Studies show that up to two-thirds of patients referred to MS specialists are ultimately determined not to have MS.[ref]
Types of MS:
Subtypes of multiple sclerosis include:[ref]
- Relapsing-remitting MS – either active or worsening
- Primary progressive – either active or with progressions
- Secondary progressive MS – can be active or with progression
At the heart of multiple sclerosis: Immune system activation
The damage to the nervous system in MS seems to be two processes:
- demyelination of the neurons
- along with progressive neurodegeneration
In MS, the innate and adaptive immune response is activated at different stages of the disease.
In MS patients, lab tests showed increased Th17 (T helper 17) in the blood and the central nervous system. Th17 cells produce inflammatory cytokines (mainly IL17). The increase in inflammatory cytokines opens up the blood-brain barrier, allowing even more inflammatory cells into the brain.[ref]
The cause of the excessive immune system activation isn’t entirely understood yet. As I’ll explain below, risk factors include changes to the gut microbiome and viral infections.
What causes demyelination of the neurons in MS?
Doctors have known since the late 1800s that lesions in the brain were at the heart of MS.
More recently, researchers have been able to untangle the reason for demyelination in the neurons, causing the ‘lesions’ showing up on MRIs and autopsies.
Myelin is the sheath covering the axons of neurons to increase the speed at which the signal travels. I always think of it like insulation over a high-quality cable.
In multiple sclerosis, inflammatory cytokines chronically attack cells in the central nervous system. MS causes a loss of the myelin sheath, which then causes a loss of function in the neurons and, eventually, neuronal death.
Microglia, which are immune cells in the brain, are persistently activated in people with MS. Additionally, during the active phase of MS, T helper 17 (Th17) cells release inflammatory cytokines.
Changes in mitochondrial energy production are key to the breakdown of the myelin sheath.
It gets a little complicated — stick with me here (or skip ahead :-).
For a more in-depth explanation, read this.
Mitochondria are the organelles in cells that produce most of the ATP for energy. Normally, cells break down glucose in the cytosol to produce a little ATP. Then, pyruvate is used in the mitochondria to produce a lot of ATP.
In people with MS, the neurons convert the pyruvate into lactate in the cytosol of the cell (the Warburg effect). The excess lactate production induces dysfunction in the neuron and causes damage.[ref]
PPAR-gamma is a transcription factor that regulates glucose metabolism. In MS, there is a downregulation of PPAR-gamma, causing a decrease in insulin sensitivity and an increase in neuroinflammation.
Researchers think the breakdown of the myelin sheath is due to abnormalities in the circadian rhythm in the neurons. Dysregulation of the circadian rhythm stimulates the WNT pathway to break down the myelin. PPAR-gamma influences the transcription of some circadian rhythm genes.
Recapping all of this:
- Inflammation due to immune system activation causes changes in the neurons.
- One change is a shift in how the neurons create energy. Instead of mitochondrial energy production, there is a shift to relying on glycolysis in the cells.
- Excessive lactate is produced in the cells.
- This shifts cells to induce T cell inflammation.
- Downregulation of PPAR-gamma decreases insulin sensitivity in the neurons, exacerbating the problem.
- Circadian rhythm abnormalities are also causing more problems, in part due to PPAR-gamma regulation.
What makes up the myelin sheath?
Key to the nerve cell damage in MS is the breakdown of myelin surrounding the axon of the neurons.
The myelin around the axons is made up of lipids (fats) such as cholesterol, phospholipids, and ceramides.
Some research points to ceramides and alterations in lipid balance in the destruction of the myelin. Additionally, oxidized sterols in the myelin sheath may also play a role in inflammation.
MS risk factors: Genes + Environment
So far, we have an inflammatory action taking place in the brain, initiated by Th17 activation. The neurons (nerve cells) have altered energy production, mitochondrial changes, circadian rhythm disruption, and links to altered lipids (fatty acids).
But what causes one person to get MS and another person to be fine?
Researchers have identified several risk factors that play a role in who is likely to get MS.
- Genetic risk factors
- Smoking and Obesity
- Vitamin D deficiency
- Epstein-Barr virus
- Gut microbiome
Environment and Lifestyle risk factors
In addition to multiple sclerosis genes (which I’ll explain in depth below), lifestyle and environmental factors have been identified that impact the risk of getting MS.
Biological sex
Women are at more than twice the risk of MS compared to men. Additionally, women tend to have more frequent relapses than men. There are theories that estrogen plays a role, but research isn’t definitive yet.[ref] Estrogen is a hormone that acts on receptors to increase the production of growth-related factors.
Circadian rhythm disruption
A disrupted circadian rhythm is linked to an increased risk of multiple sclerosis.
Research shows:
- Working the night shift at a younger age is linked to an increased relative risk of MS.[ref]
- Genetic variants in the core circadian genes are linked to increased MS risk.[ref]
- There are alterations to the circadian rhythm of nitric oxide metabolites, activity timing, HPA axis, and sleep disorders in patients with MS.[ref][ref]
Circadian rhythm is the 24-hour rhythm controlling many cellular functions. From sleep to digestion to hormone rhythms, we now know the core circadian rhythm genes indirectly control about 40% of cellular processes. Circadian rhythm also controls mitochondrial function.[ref]
Melatonin is one component of your circadian rhythm and helps keep circadian rhythm genes on track each day.
The pineal gland produces large amounts of melatonin at night – due to the lack of light in the blue spectrum. Melatonin is more than just a ‘sleep hormone’. It is essential in balancing the immune response and in fighting inflammation inside cells.
In people with MS, studies show melatonin secretion is impaired. Moreover, animal studies show that melatonin may be a key component in preventing MS.[ref]
Smoking
One of the first risk factors identified for MS was cigarette smoking.
Overall, 20% of MS cases are attributable to smoking. Among people with a specific genetic background (HLA-DRB1*15), smoking is attributed to 41% of cases.[ref]
Smoking is a risk factor for many diseases due to the increase in oxidative stress due to chronic exposure to cigarette toxicants.
Epstein-Barr virus (EBV)
Epstein-Barr virus is a ubiquitous virus — found in about 90% of adults. It causes mononucleosis (mono) if you get it as a teen. If you get it as a younger child, it can cause mild, cold-like symptoms or be asymptomatic.
Recently, a study involving US Army personnel found that the individuals who converted to Epstein Barr seropositivity (showing they had been infected) were at a 32-fold risk of MS diagnosis compared to Army personnel who were seronegative for EBV.[ref]
A viral link can’t be ruled out, either. There is quite a bit of evidence that the Epstein-Barr virus is linked in some way to MS. Some research points to EBV-infected B cells in the central nervous system. Having had a serious case of mono as a teen increases the relative risk of MS also.[ref]
However, some people who have never had EBV still get MS, so the Epstein-Barr virus isn’t the only factor causing MS.
Human Herpesvirus 6
The other virus linked to MS by a lot of research is human herpesvirus 6 (HHV-6). Similar to Epstein-Barr, HHV-6 is a ubiquitous virus that most people have in their bodies, and it can be reactivated from the laten state. Researchers find HHV-6 in MS lesions, and some researchers belive that it plays a pathogenic role in MS. [ref]
Low vitamin D
One of the first things epidemiologists noticed about multiple sclerosis is that more cases are diagnosed in the northern latitudes where there isn’t as much sunlight.
This led to the theory that low vitamin D due to a lack of sunlight is a cause of multiple sclerosis.[ref][ref]
Vitamin D is a hormone that regulates the expression of other genes, including a bunch of genes involved in the immune system.
The active form of vitamin D (1,25(OH)2D) binds to receptors in the cell nucleus, causing certain genes to be turned on for transcription. This is how vitamin D influences immune system cells and neurons. For example, vitamin D helps the innate immune system produce proteins needed for fighting pathogens. Vitamin D also increases anti-inflammatory T-regulatory cells and IL-10 producing cells.[ref]
People with MS are likely to have lower levels of the bioactive 1,25(OH)2D form of vitamin D.[ref]
Low vitamin D also ties to one of the strongest genetic components of MS.
Let me explain…
The HLA genes code for the major histocompatibility complex (MHC) proteins which are an integral part of our immune system. HLA-DRB1*1501 is a variant linked to a higher risk of MS, with studies estimating that people who are homozygous for HLA-DRB1*1501 have up to 6 times the normal risk for MS.
Vitamin D regulates the expression of HLA-DRB1. From a 2009 study on the topic: “It was found that vitamin D specifically interacts with HLA-DRB11501 to influence its expression. This study, therefore, provides more direct support for the already strong epidemiological evidence implicating sunlight and vitamin D in the determination of MS risk, and implies that vitamin D supplementation at critical time periods may be key to disease prevention.”[ref]
Gut microbiome and immune system activation
The microbes in your intestines impact your immune system in multiple ways.
Several studies have looked at the differences in the gut microbiome in people with MS compared to relatives in their households or their twin sibling.
A couple of studies show MS patients have significantly increased levels of Akkermansia muciniphila compared to household members or even twin siblings without MS.[ref]
This is interesting because Akkermansia muciniphila is usually considered beneficial in gut bacteria. This bacteria converts the mucus lining the intestines into short-chain fatty acids. But it may be that too much Akkermansia causes too much intestinal mucus degradation.[ref]
Researchers took the gut microbiome results one step further to see if the gut microbes could cause MS. Sure enough… Transferring the gut microbes from MS patients into transgenic mice induces autoimmunity at a higher rate than normal.[ref]
One study found: “Microbiome composition, function, and derived metabolites also differed in response to disease-modifying treatments. The therapeutic activity of interferon-β may in part be associated with upregulation of short-chain fatty acid transporters. Distinct microbial networks were observed in untreated MS and healthy controls. These results strongly support specific gut microbiome associations with MS risk, course and progression, and functional changes in response to treatment.”[ref]
Metabolomics studies
Measuring the concentration of metabolites can help researchers determine which biological pathways are different in a disease. Big metabolomics studies look at more than 1,000 metabolites, linking the data back to different pathways and symptoms.
For multiple sclerosis, metabolomics studies using blood samples showed an increase in “uric acid, creatinine, hypoxanthine, xanthine, uridine, β-pseudouridine, malondialdehyde, nitrite, and nitrate, along with a decrease in ascorbic acid,” compared to a healthy control group. Lipid biomarkers were also altered, including ALA and linoleic acid pathways.[ref]
Importantly, the lipid biomarkers point towards decreased pro-resolving mediators.[ref]
Other studies also showed low or non-existent levels of certain pro-resolving lipid mediators, which are essential to the resolution of inflammation.[ref]
I’ll circle back to the role of specialized pro-resolving mediators (SPMs) in the Lifehacks section below. You can also read more on the resolution of inflammation.
Demyelination and obesity
Phagocytes, a type of immune cell that engulfs and removes damaged cells, increase in diseases affecting neurons, such as multiple sclerosis. Phagocytes help remove damaged myelin for repair.
A new study points to SCD1 playing a key role in this process. Higher levels of SCD1 cause an increase in monounsaturated fatty acids, which, in the case of demyelination diseases such as MS, cause a damaging foam cell to form. These foamy phagocytes are unable to clear out the damaged myelin, which prevents the neurons from being able to regenerate.[ref]
SCD1 is also linked to obesity and altered levels of saturated vs. unsaturated fatty acids.
Related article: SCD1 genes
Gut ROS, circadian rhythm, and inflammation
It struck me when reading the research on MS that I had recently read another fascinating study on the intersection of circadian rhythm, inflammation, and the gut.
In the study, the researchers were trying to figure out why lack of sleep kills you. Fun fact: lack of sleep will kill you faster than lack of food…
The researchers used a couple of different animal models to tease out why a lack of sleep causes death. The neat part of the study was that the researchers looked at everything – they had no preconceived theory on why sleep deprivation kills.
They found that sleep deprivation causes alterations in circadian rhythm — leading to an accumulation of reactive oxygen species (ROS) in the gut. The high ROS levels in the gut caused death.
To prove this, the researchers gave oral antioxidants (vitamin C and melatonin) to the animals, and it prevented death from lack of sleep.[ref]
My point here? Disruption of circadian rhythm is powerful. Excess ROS is deadly to cells. I’ll go into more detail in the Lifehacks section on how to counteract circadian disruption and the role of melatonin both in circadian rhythm and as an intracellular antioxidant.
Mast cells:
Mast cells are part of the innate immune system. They release inflammatory payloads when activated. While commonly thought of in allergic reactions, mast cells have also been studied in the brain in MS. Animal models of MS show that mast cells likely contribute to MS. Mast cell activation can disrupt the blood-brain barrier, and mast cell activation also recruits inflammatory cells to the CNS.
Mast cells are abundant in the gut and are involved in regulating the intestinal barrier integrity. This would tie together the gut microbiome, leakiness of the blood-brain barrier, and inflammation in the central nervous system.[ref]
Genetic risk factors for MS
Genes alone don’t cause MS, but genetic variants make a big difference in who will be susceptible to MS.
Let me explain…
Twin studies show both genetic and environmental factors contribute to who gets multiple sclerosis. A study of identical twins found that only 30-35% of twin pairs both had MS.[ref]
In other words, about two-thirds of the time, only one twin would get MS, and the other would not. This indicates an environmental trigger must be involved along with genetics.
So far, researchers have identified more than a hundred genetic variants that increase or decrease the risk of MS a little bit.
Why study the genetics of MS?
The goal of identifying genes impacting MS risk is to understand (and target) the underlying pathways involved in MS.
Instead of just treating symptoms, researchers hope to develop ways of preventing MS and treating the root causes.[ref]
The HLA system
Dotting the surface of cells are proteins that let the immune system know what is going on in the cells. These antigens help the immune system know which cells are ‘self’ and which cells are ‘foreign’.
The HLA genes encode glycoprotein antigens. There are lots of genetic variants in the HLA system. Having variety in this part of the immune system means that even when new viruses or bacteria are encountered, at least part of a population will be able to fight them off.
Identifying foreign proteins is essential – but so is correctly identifying your own proteins as ‘self’ so that they aren’t attacked.
Different HLA risk alleles are linked to susceptibility to many different autoimmune diseases, such as celiac disease. For MS, an HLA type was one of the first genetic components identified as adding to susceptibility.
Beyond HLA:
Many genetic variants outside the HLA system that increase or decrease the risk of multiple sclerosis are immune system related genes. It makes sense that an overactive immune or inflammation response would be involved. For example, CD58 is a protein on the surface of antigen presenting cells, which can bind to and activate T-cells and natural killer cells to cause inflammation. Genetic variants in CD58 are linked to increased relative risk of MS.
Interestingly, researchers have also identified circadian rhythm and lipid biosynthesis genes that increase the risk of MS.
All of these genetic pathways tie into the identified causes of MS.
Multiple Sclerosis Genotype Report
Genetic variants combine with lifestyle factors and exposures in determining susceptibility to MS. Below are some of the variants identified in MS research studies.
What does your genetic risk mean?
An increase in the relative risk of MS doesn’t mean that you will necessarily get MS. Keep in mind that researchers still have a lot of questions about environmental triggers of MS – from viruses to circadian rhythm to the gut microbiome. Doing the math: If the prevalence of MS in your country is 1% and you are at a 3-fold increased risk, then you would be at a 3- in 100-lifetime risk of MS. But, I don’t think the math is that simple here. For example, if there is a specific environmental trigger that you haven’t been exposed to, then perhaps your risk is really zero.
Members: Log in to see your data below.
Not a member? Join here.
Why is this section is now only for members? Here’s why…
Lifehacks: Improving MS
My goal here is to present MS research on options that may help improve the quality of life for MS patients. Additionally, if you are worried about your own risk factors and preventing MS, you may be interested in the dietary links explained below.
A genetic picture emerges of immune response to Epstein-Barr, excess inflammation, dietary omega 6 and omega 3 consumption, the gut microbiome, short-chain fatty acid production, and circadian rhythm disruption.
Keep in mind that this section is based only on research studies — and those studies weren’t conducted on you. If you have questions on diet or supplements, please talk with your doctor and do your own due diligence. If you are on medications, talk with your doctor or pharmacist before starting any supplements to make sure there won’t be any interactions.
Pro-resolving Lipid Mediators: Resolving Inflammation
The rest of this article contains information on natural supplements, SPMs, circadian rhythm, and diets that have been researched for MS. It is for Genetic Lifehacks members only.Consider joining today to see the rest of this article.
Related Articles and Topics:
Melatonin: Key to health and longevity
Melatonin is more than just a sleep hormone. Find out why this hormone is essential for good health.
Tryptophan: Kyurenine or Serotonin/Melatonin
Your genes impact whether your cells are more likely to produce kynurenine or serotonin/melatonin from tryptophan.
Chronic Inflammation: Genetic connections
Inflammatory genetic variants can increase the risk of chronic diseases caused by elevated inflammatory cytokines.
References:
Adamczyk-Sowa, M., et al. “Influence of Melatonin Supplementation on Serum Antioxidative Properties and Impact of the Quality of Life in Multiple Sclerosis Patients.” Journal of Physiology and Pharmacology: An Official Journal of the Polish Physiological Society, vol. 65, no. 4, Aug. 2014, pp. 543–50.
Álvarez-Sánchez, Nuria, et al. “Melatonin Reduces Inflammatory Response in Peripheral T Helper Lymphocytes from Relapsing-Remitting Multiple Sclerosis Patients.” Journal of Pineal Research, vol. 63, no. 4, Nov. 2017. PubMed, https://doi.org/10.1111/jpi.12442.
Andreu-Caravaca, Luis, et al. “Can Strength Training Modify Voluntary Activation, Contractile Properties and Spasticity in Multiple Sclerosis?: A Randomized Controlled Trial.” Physiology & Behavior, vol. 255, Oct. 2022, p. 113932. PubMed, https://doi.org/10.1016/j.physbeh.2022.113932.
Baranzini, Sergio E. “Revealing the Genetic Basis of Multiple Sclerosis: Are We There Yet?” Current Opinion in Genetics & Development, vol. 21, no. 3, June 2011, pp. 317–24. PubMed Central, https://doi.org/10.1016/j.gde.2010.12.006.
Berer, Kerstin, et al. “Gut Microbiota from Multiple Sclerosis Patients Enables Spontaneous Autoimmune Encephalomyelitis in Mice.” Proceedings of the National Academy of Sciences of the United States of America, vol. 114, no. 40, Oct. 2017, pp. 10719–24. PubMed Central, https://doi.org/10.1073/pnas.1711233114.
Bjornevik, Kjetil, et al. “Longitudinal Analysis Reveals High Prevalence of Epstein-Barr Virus Associated with Multiple Sclerosis.” Science (New York, N.Y.), vol. 375, no. 6578, Jan. 2022, pp. 296–301. PubMed, https://doi.org/10.1126/science.abj8222.
Brenton, J. Nicholas, et al. “Phase II Study of Ketogenic Diets in Relapsing Multiple Sclerosis: Safety, Tolerability and Potential Clinical Benefits.” Journal of Neurology, Neurosurgery, and Psychiatry, vol. 93, no. 6, June 2022, pp. 637–44. PubMed, https://doi.org/10.1136/jnnp-2022-329074.
Cebrián-Prats, Anna, et al. “The Role of Acetylated Cyclooxygenase-2 in the Biosynthesis of Resolvin Precursors Derived from Eicosapentaenoic Acid.” Organic & Biomolecular Chemistry, vol. 20, no. 6, Feb. 2022, pp. 1260–74. PubMed, https://doi.org/10.1039/d1ob01932e.
Comabella, Manuel, et al. “Identification of a Novel Risk Locus for Multiple Sclerosis at 13q31.3 by a Pooled Genome-Wide Scan of 500,000 Single Nucleotide Polymorphisms.” PLoS ONE, vol. 3, no. 10, Oct. 2008, p. e3490. PubMed Central, https://doi.org/10.1371/journal.pone.0003490.
Cree, Bruce A. C., et al. “A Major Histocompatibility Class I Locus Contributes to Multiple Sclerosis Susceptibility Independently from HLA-DRB1*15:01.” PloS One, vol. 5, no. 6, June 2010, p. e11296. PubMed, https://doi.org/10.1371/journal.pone.0011296.
D’Cunha, Mary Anitha, et al. “CD6 Gene Polymorphism Rs17824933 Is Associated with Multiple Sclerosis in Indian Population.” Annals of Indian Academy of Neurology, vol. 19, no. 4, 2016, pp. 491–94. PubMed Central, https://doi.org/10.4103/0972-2327.192384.
de Goede, Paul, et al. “Circadian Rhythms in Mitochondrial Respiration.” Journal of Molecular Endocrinology, vol. 60, no. 3, Apr. 2018, pp. R115–30. PubMed, https://doi.org/10.1530/JME-17-0196.
De Jager, Philip L., et al. “Meta-Analysis of Genome Scans and Replication Identify CD6, IRF8 and TNFRSF1A as New Multiple Sclerosis Susceptibility Loci.” Nature Genetics, vol. 41, no. 7, July 2009, pp. 776–82. PubMed, https://doi.org/10.1038/ng.401.
De Silvestri, A., et al. “The Involvement of HLA Class II Alleles in Multiple Sclerosis: A Systematic Review with Meta-Analysis.” Disease Markers, vol. 2019, 2019, p. 1409069. PubMed, https://doi.org/10.1155/2019/1409069.
Drake, Marcus J., et al. “Results of a Randomized, Double Blind, Placebo Controlled, Crossover Trial of Melatonin for Treatment of Nocturia in Adults with Multiple Sclerosis (MeNiMS).” BMC Neurology, vol. 18, no. 1, Aug. 2018, p. 107. PubMed, https://doi.org/10.1186/s12883-018-1114-4.
Duscha, Alexander, et al. “Propionic Acid Shapes the Multiple Sclerosis Disease Course by an Immunomodulatory Mechanism.” Cell, vol. 180, no. 6, Mar. 2020, pp. 1067-1080.e16. PubMed, https://doi.org/10.1016/j.cell.2020.02.035.
Farez, Mauricio F., et al. “Anti-Inflammatory Effects of Melatonin in Multiple Sclerosis.” BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology, vol. 38, no. 10, Oct. 2016, pp. 1016–26. PubMed, https://doi.org/10.1002/bies.201600018.
Fimland, Marius S., et al. “Enhanced Neural Drive after Maximal Strength Training in Multiple Sclerosis Patients.” European Journal of Applied Physiology, vol. 110, no. 2, Sept. 2010, pp. 435–43. PubMed, https://doi.org/10.1007/s00421-010-1519-2.
Granja-Domínguez, Anabel, et al. “Effects of Pulsed Electromagnetic Field Therapy on Fatigue, Walking Performance, Depression, and Quality of Life in Adults with Multiple Sclerosis: A Randomized Placebo-Controlled Trial.” Brazilian Journal of Physical Therapy, vol. 26, no. 5, 2022, p. 100449. PubMed, https://doi.org/10.1016/j.bjpt.2022.100449.
Hedström, A. K., et al. “Smoking Is a Major Preventable Risk Factor for Multiple Sclerosis.” Multiple Sclerosis (Houndmills, Basingstoke, England), vol. 22, no. 8, July 2016, pp. 1021–26. PubMed, https://doi.org/10.1177/1352458515609794.
Hedström, Anna Karin, et al. “Shift Work at Young Age Is Associated with Increased Risk for Multiple Sclerosis.” Annals of Neurology, vol. 70, no. 5, Nov. 2011, pp. 733–41. PubMed, https://doi.org/10.1002/ana.22597.
Houen, Gunnar, et al. “Epstein-Barr Virus and Multiple Sclerosis.” Frontiers in Immunology, vol. 11, Dec. 2020, p. 587078. PubMed Central, https://doi.org/10.3389/fimmu.2020.587078.
iMSMS Consortium. Electronic address: sergio.baranzini@ucsf.edu and iMSMS Consortium. “Gut Microbiome of Multiple Sclerosis Patients and Paired Household Healthy Controls Reveal Associations with Disease Risk and Course.” Cell, vol. 185, no. 19, Sept. 2022, pp. 3467-3486.e16. PubMed, https://doi.org/10.1016/j.cell.2022.08.021.
International Multiple Sclerosis Genetics Consortium. “The Genetic Association of Variants in CD6, TNFRSF1A and IRF8 to Multiple Sclerosis: A Multicenter Case-Control Study.” PloS One, vol. 6, no. 4, Apr. 2011, p. e18813. PubMed, https://doi.org/10.1371/journal.pone.0018813.
Kanabrocki, E. L., et al. “Altered Circadian Relationship between Serum Nitric Oxide, Carbon Dioxide, and Uric Acid in Multiple Sclerosis.” Chronobiology International, vol. 21, no. 4–5, July 2004, pp. 739–58. PubMed, https://doi.org/10.1081/cbi-200025981.
Langer-Gould, Annette, et al. “Seafood, Fatty Acid Biosynthesis Genes and Multiple Sclerosis Susceptibility.” Multiple Sclerosis (Houndmills, Basingstoke, England), vol. 26, no. 12, Oct. 2020, pp. 1476–85. PubMed Central, https://doi.org/10.1177/1352458519872652.
Lavtar, Polona, et al. “Association of Circadian Rhythm Genes ARNTL/BMAL1 and CLOCK with Multiple Sclerosis.” PLoS ONE, vol. 13, no. 1, Jan. 2018, p. e0190601. PubMed Central, https://doi.org/10.1371/journal.pone.0190601.
Lee, Jennifer E., et al. “A Modified MCT-Based Ketogenic Diet Increases Plasma β-Hydroxybutyrate but Has Less Effect on Fatigue and Quality of Life in People with Multiple Sclerosis Compared to a Modified Paleolithic Diet: A Waitlist-Controlled, Randomized Pilot Study.” Journal of the American College of Nutrition, vol. 40, no. 1, Jan. 2021, pp. 13–25. PubMed, https://doi.org/10.1080/07315724.2020.1734988.
Liu, Jiahe, et al. “Association of EVI5 Rs11808092, CD58 Rs2300747, and CIITA Rs3087456 Polymorphisms with Multiple Sclerosis Risk: A Meta-Analysis.” Meta Gene, vol. 9, Apr. 2016, pp. 97–103. PubMed Central, https://doi.org/10.1016/j.mgene.2016.04.005.
Maier, Lisa M., et al. “IL2RA Genetic Heterogeneity in Multiple Sclerosis and Type 1 Diabetes Susceptibility and Soluble Interleukin-2 Receptor Production.” PLOS Genetics, vol. 5, no. 1, Jan. 2009, p. e1000322. PLoS Journals, https://doi.org/10.1371/journal.pgen.1000322.
Morowitz, Michael J., et al. “Contributions of Intestinal Bacteria to Nutrition and Metabolism in the Critically Ill.” The Surgical Clinics of North America, vol. 91, no. 4, Aug. 2011, pp. 771–85. PubMed Central, https://doi.org/10.1016/j.suc.2011.05.001.
Motaghi, Najmeh, et al. “Lavender Improves Fatigue Symptoms in Multiple Sclerosis Patients: A Double-Blind, Randomized Controlled Trial.” Multiple Sclerosis and Related Disorders, vol. 65, Sept. 2022, p. 104000. PubMed, https://doi.org/10.1016/j.msard.2022.104000.
MS & Vitamin D Deficiency | Overcoming Multiple Sclerosis. https://overcomingms.org/recovery-program/sunlight-vitamin-d. Accessed 7 Dec. 2022.
Najafi, Mohammad Reza, et al. “Circadian Rhythm Sleep Disorders in Patients with Multiple Sclerosis and Its Association with Fatigue: A Case-Control Study.” Journal of Research in Medical Sciences: The Official Journal of Isfahan University of Medical Sciences, vol. 18, no. Suppl 1, Mar. 2013, pp. S71-73.
Orefice, Nicola S., et al. “Oral Palmitoylethanolamide Treatment Is Associated with Reduced Cutaneous Adverse Effects of Interferon-Β1a and Circulating Proinflammatory Cytokines in Relapsing–Remitting Multiple Sclerosis.” Neurotherapeutics, vol. 13, no. 2, Apr. 2016, pp. 428–38. PubMed Central, https://doi.org/10.1007/s13311-016-0420-z.
Patsopoulos, Nikolaos A. “Genetics of Multiple Sclerosis: An Overview and New Directions.” Cold Spring Harbor Perspectives in Medicine, vol. 8, no. 7, July 2018, p. a028951. perspectivesinmedicine.cshlp.org, https://doi.org/10.1101/cshperspect.a028951.
Platero, Jose Luis, et al. “The Impact of Coconut Oil and Epigallocatechin Gallate on the Levels of IL-6, Anxiety and Disability in Multiple Sclerosis Patients.” Nutrients, vol. 12, no. 2, Jan. 2020, p. 305. PubMed, https://doi.org/10.3390/nu12020305.
Pröbstel, Anne-Katrin, and Sergio E. Baranzini. “The Role of the Gut Microbiome in Multiple Sclerosis Risk and Progression: Towards Characterization of the ‘MS Microbiome.’” Neurotherapeutics, vol. 15, no. 1, Jan. 2018, pp. 126–34. PubMed Central, https://doi.org/10.1007/s13311-017-0587-y.
Prüss, Harald, et al. “Proresolution Lipid Mediators in Multiple Sclerosis — Differential, Disease Severity-Dependent Synthesis — A Clinical Pilot Trial.” PLoS ONE, vol. 8, no. 2, Feb. 2013, p. e55859. PubMed Central, https://doi.org/10.1371/journal.pone.0055859.
Ramagopalan, Sreeram V., et al. “Expression of the Multiple Sclerosis-Associated MHC Class II Allele HLA-DRB1*1501 Is Regulated by Vitamin D.” PLoS Genetics, vol. 5, no. 2, Feb. 2009, p. e1000369. PubMed Central, https://doi.org/10.1371/journal.pgen.1000369.
Sánchez-López, Angélica L., et al. “Efficacy of Melatonin on Serum Pro-Inflammatory Cytokines and Oxidative Stress Markers in Relapsing Remitting Multiple Sclerosis.” Archives of Medical Research, vol. 49, no. 6, Aug. 2018, pp. 391–98. PubMed, https://doi.org/10.1016/j.arcmed.2018.12.004.
Sawcer, Stephen, et al. “Genetic Risk and a Primary Role for Cell-Mediated Immune Mechanisms in Multiple Sclerosis.” Nature, vol. 476, no. 7359, Aug. 2011, pp. 214–19. PubMed Central, https://doi.org/10.1038/nature10251.
Schott, Geraldine, et al. “U2AF2 Binds IL7R Exon 6 Ectopically and Represses Its Inclusion.” RNA (New York, N.Y.), vol. 27, no. 5, Feb. 2021, pp. 571–83. PubMed, https://doi.org/10.1261/rna.078279.120.
Sedaghat, Fatemeh, et al. “Mediterranean Diet Adherence and Risk of Multiple Sclerosis: A Case-Control Study.” Asia Pacific Journal of Clinical Nutrition, vol. 25, no. 2, 2016, pp. 377–84. PubMed, https://doi.org/10.6133/apjcn.2016.25.2.12.
Silva, Tamiris, et al. “Effect of Photobiomodulation on Fatigue in Individuals with Relapsing-Remitting Multiple Sclerosis: A Pilot Study.” Lasers in Medical Science, vol. 37, no. 8, Oct. 2022, pp. 3107–13. PubMed, https://doi.org/10.1007/s10103-022-03567-3.
Silva, Ygor Parladore, et al. “The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication.” Frontiers in Endocrinology, vol. 11, 2020. Frontiers, https://www.frontiersin.org/articles/10.3389/fendo.2020.00025.
Simon, Kelly Claire, et al. “Genetic Predictors of 25-Hydroxyvitamin D Levels and Risk of Multiple Sclerosis.” Journal of Neurology, vol. 258, no. 9, Sept. 2011, pp. 1676–82. PubMed, https://doi.org/10.1007/s00415-011-6001-5.
Skarlis, Charalampos, and Maria Anagnostouli. “The Role of Melatonin in Multiple Sclerosis.” Neurological Sciences: Official Journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology, vol. 41, no. 4, Apr. 2020, pp. 769–81. PubMed, https://doi.org/10.1007/s10072-019-04137-2.
Swaminathan, Bhairavi, et al. “Validation of the CD6 and TNFRSF1A Loci as Risk Factors for Multiple Sclerosis in Spain.” Journal of Neuroimmunology, vol. 223, no. 1–2, June 2010, pp. 100–03. PubMed, https://doi.org/10.1016/j.jneuroim.2010.03.020.
Wahls, Terry L., et al. “Impact of the Swank and Wahls Elimination Dietary Interventions on Fatigue and Quality of Life in Relapsing-Remitting Multiple Sclerosis: The WAVES Randomized Parallel-Arm Clinical Trial.” Multiple Sclerosis Journal – Experimental, Translational and Clinical, vol. 7, no. 3, July 2021, p. 205521732110353. DOI.org (Crossref), https://doi.org/10.1177/20552173211035399.
Wang, L. M., et al. “Interleukin 2 Receptor α Gene Polymorphism and Risk of Multiple Sclerosis: A Meta-Analysis.” The Journal of International Medical Research, vol. 39, no. 5, 2011, pp. 1625–35. PubMed, https://doi.org/10.1177/147323001103900505.
Yosefifard, Masoomeh, et al. “A Randomized Control Trial Study to Determine the Effect of Melatonin on Serum Levels of IL-1β and TNF-α in Patients with Multiple Sclerosis.” Iranian Journal of Allergy, Asthma, and Immunology, vol. 18, no. 6, Nov. 2019, pp. 649–54. PubMed, https://doi.org/10.18502/ijaai.v18i6.2177.