Key Takeaways:
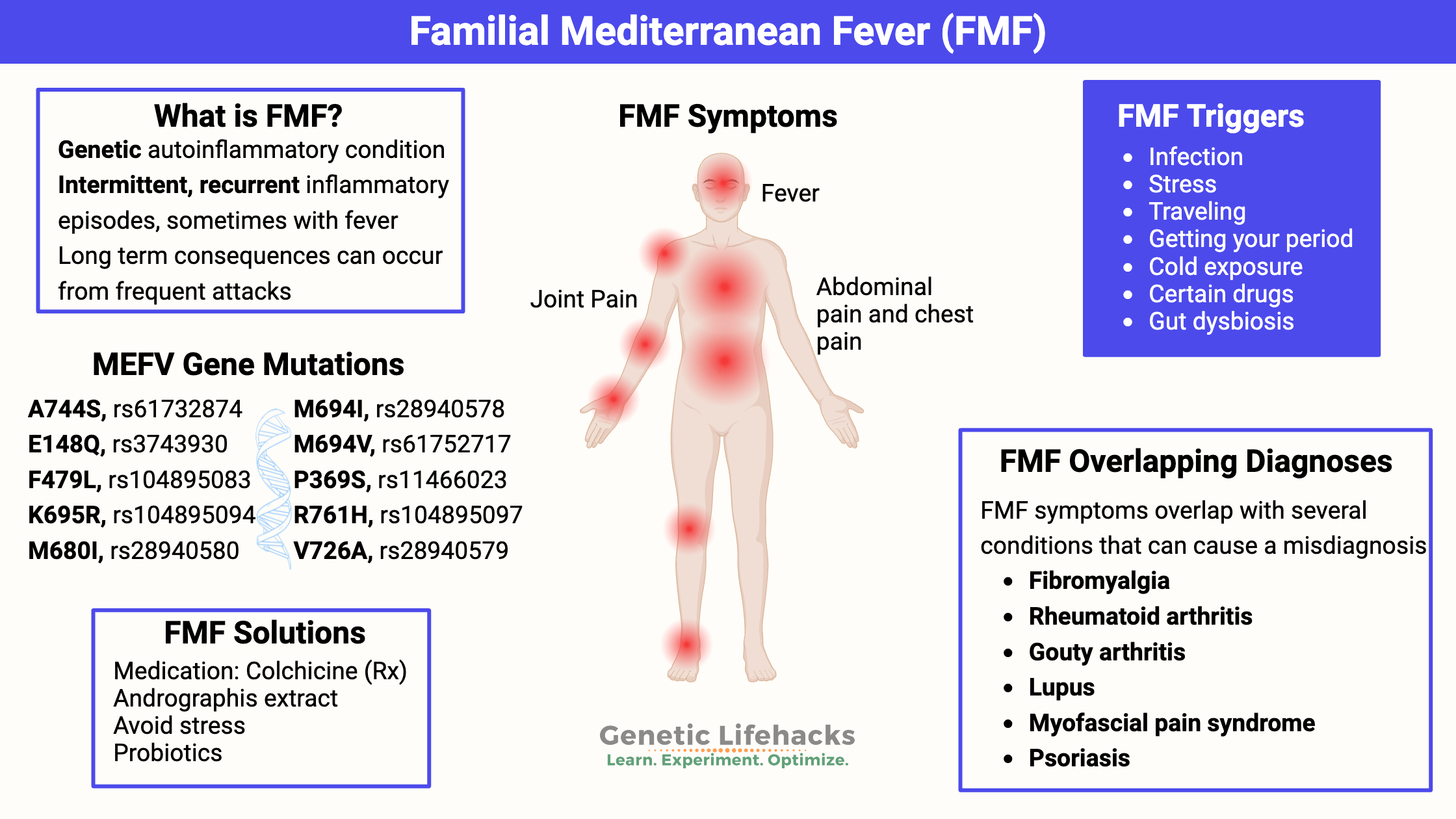
~ Familial Mediterranean fever (FMF) is a genetic autoinflammatory condition, causing episodes of painful joints, pain in the abdomen, or pain in the chest, often accompanied by a fever.
~ Mutations in the MEFV gene can cause familial Mediterranean fever, but not everyone with the mutation will have FMF.
~ FMF is often misdiagnosed as fibromyalgia, gouty arthritis, myofascial pain syndrome, lupus, or rheumatoid arthritis.
This article explains the background science of the MEFV gene, how to check your genetic raw data for MEFV variants, and possible solutions.
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Consider joining today.
What is familial Mediterranean fever (FMF)?
Familial Mediterranean fever (FMF) is a genetic autoinflammatory condition marked by recurrent episodes of fever and pain, often starting in childhood. Symptoms can include:
- fever
- inflammation and pain in a joint (often different joints for each episode)
- pain in the abdomen or chest due to inflammation
- rash, headache
This condition often shows up first in childhood with unexplained fever plus aches and pains. Sometimes the inflammation can cause a rash or headache as well as inflaming the membrane around the spinal cord or testicles.
Triggering FMF episodes:
For about half of FMF patients, the episodes are preceded by a prodromal period, such as feeling “off” and knowing that joint pain and fever or night sweats are coming.[ref]
Commonly, there is a triggering event before an episode. Triggers include:[ref]
- infection (such as getting a cold)
- stress
- traveling
- getting your period
- cold exposure
- certain drugs
FMF is a genetic, auto-inflammatory disease
FMF was classically thought of as an “autosomal recessive” genetic disease, which required the inheritance of two mutated genes, but more recent studies show that this isn’t always true.
With the recent prevalence of genetic testing, researchers find that FMF is a disease with a range of symptoms. FMF symptoms sometimes show up in those who are heterozygous (one copy) for the mutations, and there are a few cases of it not affecting those who are homozygous (2 copies).
MEFV is the gene responsible for familial Mediterranean fever. MEFV encodes a protein called pyrin, which interacts with the immune system.
Pyrin regulates interleukin-1 beta (IL1B), which is a cytokine important in inflammatory responses, including fever.[ref] Interleukin-18 (IL18) is also elevated in patients with MEFV mutations.[ref] NLRP3 is also higher in people with familial Mediterranean fever.[ref]
While there are over 310 different MEFV mutations that have been identified and linked to familial Mediterranean fever, there are a few (E148Q, M680I, M694I, M694V, and V726A – listed below) that “account for as many as 80% of FMF cases in classically affected populations”.[ref]
Results of recent research show that up to 30% of FMF cases are heterozygous (one copy) for a MEFV mutation.[ref] On the other hand, some people who carry one or two copies of the MEFV mutation have no symptoms. Researchers theorize that there may be another genetic mutation also involved – or – environmental factors.
- A recent study found that those with European ancestry usually have milder symptoms than those of Mediterranean ancestry.[ref]
- The gut microbiome may also be involved, with changes to the microbiome found in those in acute attacks.[ref]
Long-term harm from frequent attacks:
For people with frequent attacks, the long-term consequences of FMF can include amyloidosis and kidney failure, so definitely talk with your doctor if you have the genetic variants along with symptoms.
Diagnosis:
The criteria for diagnosis include recurrent fever with chest (pleuritis or periocarditis) or joint pain, fever alone, monoarthritis, and favorable response to colchicine.[ref] During an attack, blood or urine tests can show elevated inflammatory markers.
Familial Mediterranean Fever is often misdiagnosed:
Aching joints or muscles, pain in the abdomen, pain across the chest — these symptoms describe several different rheumatoid-type diagnoses. Numerous studies show an overlap of conditions with symptoms similar to FMF and missed diagnoses. It isn’t clear if the presence of the MEFV gene variants makes the original diagnosis more likely – or if patients were misdiagnosed in the first place.
A LA Times article from 2009 explains, “Even today, many patients bear the scars of needless appendectomies. Others are mistakenly diagnosed with such ailments as diverticulitis, pancreatitis, pleurisy, and lupus — even psychiatric disorders — until a light goes on and patients are finally placed on colchicine.”
Conditions that overlap with familial Mediterranean fever:
Research shows that MEFV mutations are found at higher-than-normal rates in people with the following conditions. In some cases, it may be that the condition was misdiagnosed, and FMF is the root cause. For example, gouty arthritis and fibromyalgia both have symptoms seen in familial Mediterranean fever. For others, it may be that the MEFV mutation and pyrin activation exacerbate the condition.
| Condition | Overlapping Symptoms |
|---|---|
| Fibromyalgia | Joint/muscle pain |
| Gouty arthritis | Joint pain |
| Lupus | Fever, joint pain, pleurisy |
| Rheumatoid arthritis | Joint pain, inflammation |
| Myofascial Pain Syndrome | Joint pain, inflammation |
| Pustular psoriasis | Fever, joint pain |
| Mast Cell Activation Syndrome | Inflammation |
Fibromyalgia:
A study of fibromyalgia patients and their families found that 15% carried heterozygous mutations for familial Mediterranean fever as well as having elevated levels of IL-1B (also found in FMF patients). The study theorizes that a COMT genetic variant (rs4680, A/A) may combine with the heterozygous FMF mutation to cause fibromyalgia-like symptoms.[ref]
Related articles: COMT SNPs and Fibromyalgia
Rheumatoid arthritis:
In a study of ‘asymptomatic’ parents of children with FMF, many were found to have diagnoses of rheumatoid arthritis, rheumatic fever, and arthralgia.[ref] People with palindromic rheumatism and intermittent hydrarthrosis are more likely to carry MEFV mutations.[ref]
Related article: Rheumatoid arthritis
Gouty arthritis:
In a study on gouty arthritis, 38% were found to carry an MEFV variant that can cause familial Mediterranean fever.[ref]
Lupus:
A study of lupus patients found that those carrying MEFV variants had an earlier onset of disease, more episodes of fever, and pleurisy.[ref]
Related article: Lupus
Myofascial pain syndrome:
A small study of people with myofascial pain syndrome found that 75% of them carried one copy of the MEFV mutation.[ref]
Behçet’s disease or Sweet Syndrome:
70% of patients with neurological symptoms in Behçet’s or Sweet’s Disease have MEFV mutations.[ref]
Psoriasis:
Psoriasis also links to excess inflammation and is found more commonly in people with FMF diagnosis.[ref] Pustular psoriasis is also common in people with heterozygous (single copy) MEFV mutations. This study found that a third of pustular psoriasis patients carried MEFV mutations.[ref]
Related article: Psoriasis
Vasculitis:
There is an increased incidence of vasculitis in people with MEFV mutations.[ref]
Mast cell activation syndrome (MCAS):
A recent study found that the autoinflammatory activation in FMF may activate mast cells in some patients. Mast cells are immune system cells that release histamine and tryptase. The study results showed that 70% of FMF patients had elevated histamine and tryptase levels and features of systemic mast cell activation.[ref]
Related articles: Mast cell activation syndrome and Histamine intolerance
Pyrin and inflammation:
I mentioned above that the MEFV gene encodes the pyrin protein. Pyrin is produced in neutrophils, eosinophils, and monocytes, which are immune cells that are types of white blood cells. Pyrin is part of the structure of the cytoskeleton structure of these cells, and it helps the cells to be able to interact with other molecules in inflammatory processes. Pyrin acts to keep the inflammation process under control — like a regulator to keep inflammation from going off the rails.
The familial Mediterranean fever mutations in MEFV cause malformation in pyrin, which then causes less control of inflammation.
The cytoskeleton is extremely important in immune system cells that can migrate to inflammation, activate other immune responses, and even engulf pathogens. It’s more than just a scaffolding in cells and remodels when it senses pathogens.[ref]
Pyrin can sense certain bacterial modifications within the cell and, upon detection, can initiate the assembly of the inflammasome complex, leading to the activation of caspase-1, which in turn processes pro-IL-1β into its active form. Pyrin also interacts with IL-18, another inflammatory cytokine.[ref]
The bacterial modification that pyrin senses is the interference with RhoGTPase. RhoGTPase regulates several processes inside a cell, including cytoskeleton organization. Some pathogens hijack RhoGTPase, as a way of escaping the immune response. Some of the pathogens that inactivate Rho GTPase and trigger pyrin include Bordetella pertussis, Burkholderia cenocepacia, Clostridium botulinum, Clostridium difficile, and Yersinia pestis. [ref]
Trade-offs: Surviving the plague
For many deleterious mutations that are relatively common in the population, there is a trade-off between the mutation symptoms and resistance to disease. For example, people with sickle cell anemia have resistance to malaria… Thus, people with the sickle cell mutation who live in areas with malaria are more likely to survive childhood and pass on the mutation to their children.
| Genetic Variant Effect | Evolutionary Advantage |
|---|---|
| FMF mutation (MEFV) | Resistance to Yersinia pestis (plague) |
| FMF diagnosis | 25-33% reduced risk of cancer |
Researchers have found that people with familial Mediterranean fever mutations do have a positive trade-off: resistance to Yersinia pestis, the bacterium that causes the plague.[ref][ref] Thus, it is likely that the mutations for FMF are more common in the population today because people with the mutation were more likely to have survived outbreaks of the plague.
Another positive is that people with familial Mediterranean fever diagnoses are at a 25-33% reduced risk of cancer in general. Studies show this in several population groups.[ref][ref] It is not known at this point whether the MEFV mutation decreases cancer risk or if the decrease is due to FMF patients taking anti-inflammatory drugs.[ref]
Familial Mediterranean Fever Genotype Report
Lifehacks: Natural solutions for familial Mediterranean fever
If anything above is highlighted, you should consider whether FMF could be the root cause of any periodic joint pain, night sweats, inflammatory flare-ups, or abdominal pains.
There are a couple of natural supplements that may help, and lifestyle factors that come into play.
Related Articles and Topics:
Rheumatoid Arthritis: Genetics, Root Causes, and Treatment Research