Key takeaways:
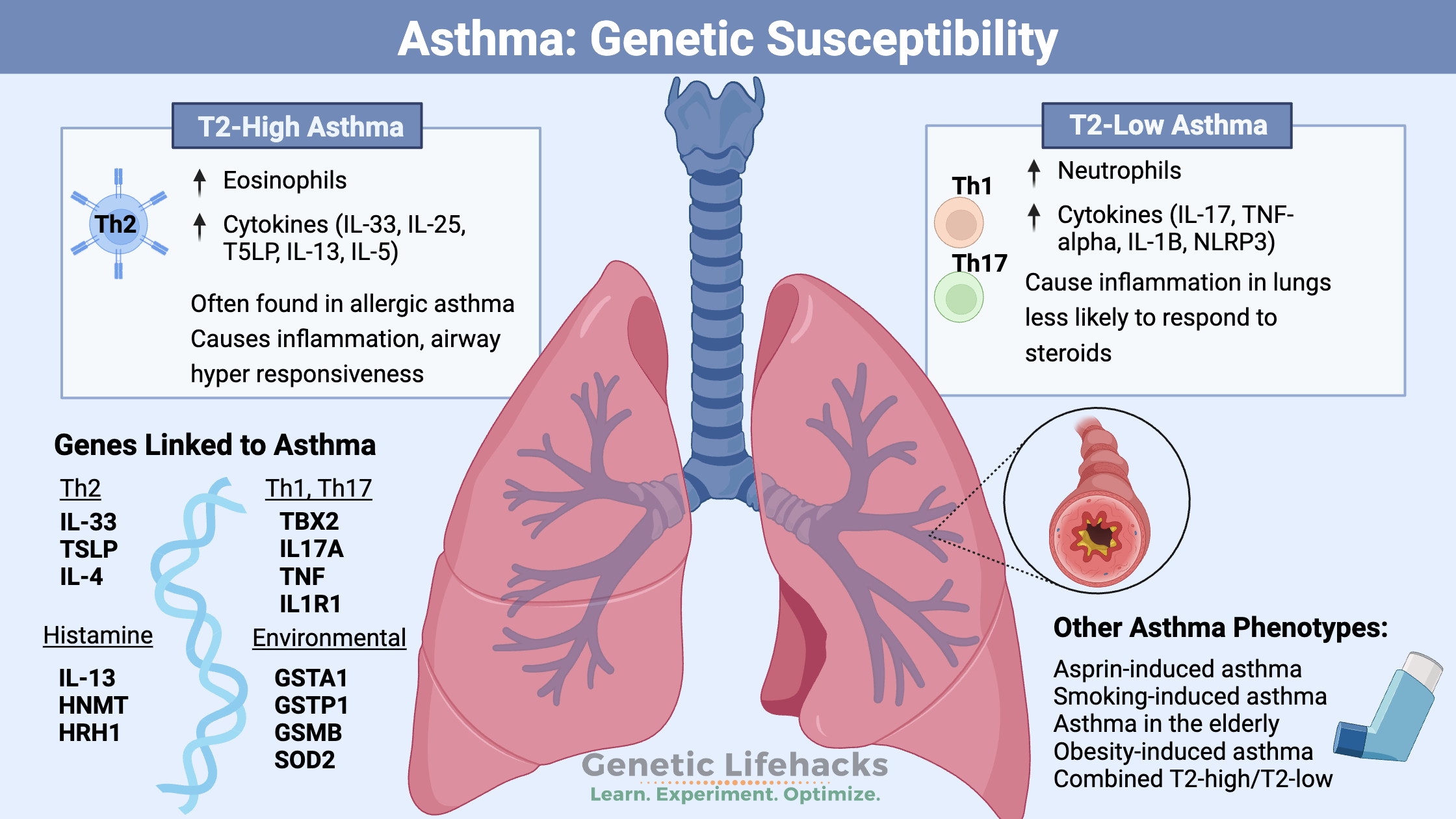
~ Asthma can be broadly categorized into T2-high and T2-low phenotypes, each characterized by different cellular and molecular mechanisms and different inflammatory cytokines.
~ Genetic variants play a big role in susceptibility to asthma, but genes alone do not cause asthma.
~ Environmental and lifestyle factors, such as exposure to allergens, pollution, smoking, obesity, and aging, significantly influence asthma phenotypes.
Asthma: A Combination of Genetic Susceptibility and Environmental Exposures
Asthma heritability is about 60%, showing that it is a combination of genetic susceptibility plus environmental exposures.[ref] Knowing your genetic variants that increase susceptibility to asthma can help you understand its root cause.
Asthma is a general term that refers to airway hyperresponsiveness – tightening or constriction of the muscles lining the lungs – along with chronic inflammation that can cause airway remodeling. There are several types of asthma, and definitions vary depending on the research study or organization involved. Asthma can be either episodic or with continual symptoms. Episodic asthma involves intermittent airway constriction which cause severe episodes of airway narrowing. [ref]
Types of Asthma:
Over the decades, the understanding of asthma has evolved as researchers have learned more about its underlying causes. For example, in the 1940s, doctors distinguished between allergic and non-allergic asthma, based on whether an allergen was likely involved in the asthma symptoms or if the asthma seemed to come from within.[ref]
Since then, a better understanding of cellular processes and lung tissue has led to better classifications based on biomarkers. The terminology in this article may be more research-based than what you hear from your doctor, but stick with me here — this dividing of asthma types by underlying pathology will make more sense of the genes involved.
T2-High Asthma:
Th2 inflammation involves T helper 2 cells, elevated eosinophils, and increased levels of certain cytokines. This type of response involves elevated levels of IL-33, IL-25, and thymic stromal lymphopoietin (TSLP), all of which increase inflammation in the lungs. People with T2-high asthma have biomarkers associated with higher levels of eosinophils as well as specific inflammatory cytokines. This is sometimes referred to as eosinophilic asthma.[ref]
- T2-high asthma can be either early-onset asthma (childhood asthma, usually allergic) or adult asthma, but more often is early-onset.
- Eosinophil levels are elevated and it usually responds to corticosteroids.
- Often due to allergens and high IgE levels.[ref]
- More frequently involves frequent or chronic rhinitis.[ref]
- T2-high asthma affects about 50% or more of patients[ref]
T2-Low Asthma:
T2-low asthma does not have significant type 2 inflammation and eosinophil levels are not elevated. This type is often less responsive to corticosteroids and may require alternative treatment options.
Instead of the Th2 pathway being activated, either the Th1 or Th17 pathways are involved. T1 immune activation is triggered in response to viral infections. Th17 is activated when IL17A or IL17F are involved, which is characterized by neutrophil recruitment to the airways and steroid resistance. T2 low can also involve NLRP3 inflammasome activation, elevated TNF-alpha, and IL-1B activity. Neutrophils may be elevated, and this is type of asthma is sometimes referred to as neutrophilic asthma. [ref][ref]
- T2-low involves Th1 or Th17 activation instead of Th2 activation.
- Neutrophils may be elevated, and it does not respond to corticosteroids as well as T2-high.
- Usually not due to allergens.
- Affects fewer than half of asthma patients.
- Gene expression studies show that mitochondrial dysfunction likely contributes to T2-low asthma.[ref]
Combinations:
Not everyone falls neatly into the T2-high or T2-low phenotypes, so there can be overlap here with some patients having multiple pathways activated. Or patients can have just some of the features, such as elevated eosinophils but without IgE allergies.
Additional asthma types based on clinical phenotypes:
You’ll also see asthma subtypes defined based on environmental or lifestyle factors rather than what’s going on with the immune system response. People with these types may also fall into the T2-high or T2-low categories.
- Aspirin-induced asthma:
This is an inflammatory response to aspirin or other COX-1 inhibitors. While not a traditional IgE allergy, it is a T2-high response that occurs in ~7% of asthma patients.[ref] - Smoking-induced asthma:
Just like it sounds – cigarette smoking can be a major cause of asthma. Smoke in the lungs causes a T2-low type of asthma that is often steroid resistant. - Obesity-related asthma:
Obesity is associated with an increased risk of non-allergic or late-onset asthma (usually T2-low). This may be due to systemically elevated inflammatory cytokines. - Asthma in the elderly:
People over the age of 65 who have asthma fall into this subtype. While this can be due to asthma that has persisted since childhood, most often ‘asthma in the elderly’ involves neutrophil infiltration of the airways and can be driven by T1 or TH17 pathways (T2-low).
Here’s a graphical overview of what is going on in the different types of asthma at a cellular level:
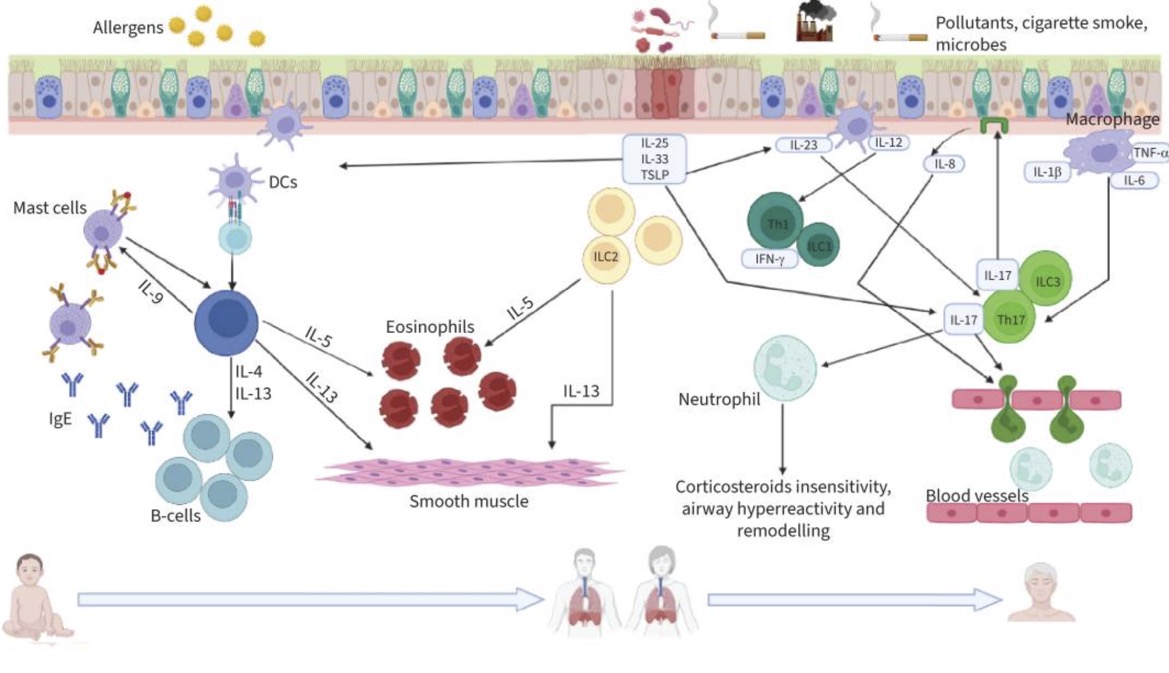
Wait – What are Th2 cells? Eosinophils?
Before we go any further, let me explain a little more about T cells and eosinophils.
T cells are a type of white blood cell that can differentiate into different types of immune cells, and there are multiple types of T cells. T helper cells (also called CD4+) are part of the adaptive immune response and help fight off pathogens.
Th2 (T helper 2) cells are a type of T helper cell activated by extracellular pathogens (outside the cell, e.g. worms). They are also called T2 cells when talking about broad immune pathways.
Th2 cells:[ref]
- release IL-13, IL-4, and IL-5, which are inflammatory cytokines
- promote allergic responses by stimulating B cells to produce more IgE antibodies
- activate and recruit eosinophils
One reason for a Th2 response is to fight off parasitic worms. The Th2 cells produce IL-13, which causes mast cells or basophils to release a bunch of histamine, serotonin, and other mediators — which in the intestines causes diarrhea to expel the worms. Throughout human history, parasites have been a big problem, so getting rid of them has been a benefit.[ref]
Th2 activation in the lungs: The lung epithelial cells can be induced to release ‘alarmins’, which include IL-33, TSLP, and IL-25. All of these then promote the Th2 response in response to lung tissue insult (e.g. viral infection, cigarette smoke, air pollution, mold spores,etc.).[ref] (You will see all of these mentioned again in the Genotype Report section below).
Eosinophils are another type of white blood cell. They usually make up about 0.5 – 1% of white blood cells. Eosinophils can release granules, including IL-8 and GM-CSF, that can destroy parasites, intracellular bacteria, and allergens. They can also produce TGF-beta, which contributes to fibrosis in the airways [ref]
Studies show that a blood eosinophil count of >3% or >300 cells/μL can predict a good response to corticosteroid treatment and also can indicate an increased risk of asthma exacerbations.[ref]
Th1 and Th17 dominate in T2-low asthma.
Th1 (T helper 1) cells:[ref]
- produce IFN-γ, TNF-alpha, IL-2, and TNF-β
- fight intracellular pathogens such as viruses and some types of bacteria
- can activate macrophages.
- T1 pathways tend to dominate in autoimmune diseases such as MS
Th17 (T helper 17) cells:
- fight extracellular bacteria and fungi (mold)
- promote inflammation and recruit neutrophils
- produce IL-17A, IL-17F, and IL-22
Related articles: IL-17A and IL-17F and TNF-alpha
Balancing immune response:
Th1 and Th2 responses will ideally counterregulate each other. For example, IFN-γ from Th1 cells inhibits Th2 differentiation, while IL-4 from Th2 cells inhibits Th1 differentiation.[ref] Thus, in healthy people without asthma or autoimmune diseases, there is a balance between the T1 and T2 immune response pathways.
To recap for asthma:
- T2-high asthma is associated with Th2 responses
- T2-low asthma may involve Th1 or Th17 responses, or other non-T2 mechanisms
Pathogens as a cause of asthma and as a trigger for exacerbation
Bacterial and viral infections may be involved in T2-low asthma as a cause or as a trigger for asthma exacerbations. Recurrent pathogen infections, especially bacterial staphylococcal infections, may cause mast cells in the lung to degranulate and trigger inflammatory responses.[ref][ref]
Research shows that rhinovirus infection, along with allergen exposure or genetic susceptibility, may play a role in the development of asthma. Rhinovirus infections are one cause of the common cold, and everyone is exposed to them regularly. When infected with a rhinovirus as a child, the lungs express Th2 pathway cytokines such as Il-13, IL-33, and TSLP.[ref]
In children, either RSV or rhinovirus infections can precede the development of asthma, and these respiratory viruses have been shown to cause wheezing. Rhinovirus is associated with the development of allergic asthma in children, and RSV is associated with non-allergic asthma.[ref]
One study found that wheezing was associated with viral detection in 100% of children and 80% of adults with asthma exacerbation. Rhinoviruses cause the common cold and can circulate year-round, with peak prevalence in early fall and spring. About 35% of people can have a rhinovirus infection with no symptoms.[ref]
Bacteria that colonize the lungs may also be involved in persistent asthma attacks or symptoms. A 2022 study found that more than 50% of people with moderate to severe asthma had lungs colonized with Haemophilus influenzae.[ref]
In people with allergic asthma, IgE (produced in response to an allergen) has been shown to inhibit the production of interferon-alpha. Interferons are produced as an initial response to fight off influenza (flu) and rhinoviruses (colds). So you have a cycle where allergic asthma reduces the ability to fight off respiratory viruses. For someone who is sensitized to pollen or mold, spring or fall when pollen or mold counts are high can then cause respiratory viruses to exacerbate asthma and cause wheezing.[ref]
Additional Environmental Factors in Asthma:
Gut and lung microbiome in childhood asthma:
The healthy lung microbiome includes Bacteroidetes, Actinobacteria, and Firmicutes bacteria. In children with asthma, differences are seen in both the lung microbiome and the gut microbiome. Part of the difference may be due to the different types of bacteria that colonize the nose and throat and the frequency of viral upper respiratory tract infections. Several studies also show that an altered gut microbiome in infants increases the risk of asthma in childhood.[ref]
Exposure to antibiotics during the first six months of life doubles the risk of asthma. Similarly, acid reflux medications taken in infancy also alter the microbiome and double the risk of asthma in childhood.[ref]
PFAS or PFOAs :
Inhalation of PFOS or PFAS has been shown in animal studies and epidemiological studies to cause inflammation and trigger IL-1B in the lungs. Animal studies also show that PFOS exposure leads to impaired epithelial barrier function.[ref][ref] This is a timely topic when coupled with the research showing that most face masks (surgical, some N95, KN95) contain PFAS and that the main route of exposure is via inhalation.[ref][ref][ref][ref]
In a study of over 500 teenagers, girls with higher PFOS levels were more likely to have problems with asthma.[ref]
Air pollution:
Exposure to particulate air pollution and other organic aerosols, except ozone, slightly increases the risk of asthma. A study of over one million children in Denmark showed that higher exposure to various forms of air pollution increased the relative risk of asthma by 1-8%[ref] While the risk of developing asthma due to air pollution is relatively small, exposure to higher levels of air pollution once asthma has developed is also associated with a 1% increase in the relative risk of emergency department visits for asthma.[ref]
IL-13 and NSAID-exacerbated respiratory disease.
Taking aspirin or NSAIDs (COX-1 inhibitors) can trigger asthma flares in about 10% of people with asthma. IL-13, which is elevated in T2-high asthma, plays a role in regulating the arachidonic acid pathway, downregulating PGE2, which then interacts with NSAIDs.[ref][ref]
Related article: IL-13, Th2, and allergies
Mold exposure:
Mold can release spores and mycotoxins, both of which can cause an immune response in the lungs. A meta-analysis of 148 studies found that mold exposure is associated with an increased risk of asthma development and of exacerbation of both allergic and non-allergic asthma.[ref] Mold sensitivity in asthma is usually diagnosed with a skin prick test to see if the individual reacts to molds, with Aspergillus fumigatus being the most common mold allergy in asthma. Mycotoxin exposure has also been shown to worsen asthma symptoms in the absence of mold allergies.[ref]
Related article: Mold and mycotoxins
Asthma, estrogen, and histamine
Estrogen receptors interact with histamine in airway smooth muscle in people with asthma. Interestingly, in children, boys have twice the risk of asthma compared to girls. In adults, however, women of reproductive age are more likely to have asthma than men. Some theorize that higher estrogen levels in women contribute to the increased risk of asthma. Let me lay out the theory – Asthma is caused by an overreaction in the smooth muscles of the airway. Intracellular calcium [Ca2+ ] levels regulate bronchoconstriction (constricted lungs, wheezing) or bronchodilation (relaxed lungs). A recent research study found that estrogens reduce calcium response to drugs that cause bronchoconstriction via an estrogen receptor (ERα). The researchers found increased estrogen receptors in the airway smooth muscle cells in people with asthma. In airway cells, exposure to a drug that binds to estrogen receptors increases the response to histamine.[ref]
Related article: Histamine and estrogen
Growing up on a farm, and Farmer’s lung:
Living on a farm and drinking raw milk is associated with a two-fold decrease in the risk of asthma in children. Traditional farming methods, such as the Amish do, are linked to a four-fold decreased risk of childhood asthma. Having a pet in the home during the first years of life is also slightly protective against childhood asthma. The thought is that exposure to bacterial diversity is helpful and that exposure to N-glycolylneuraminic acid (Neu5Gc), a sialic acid produced by non-human mammals is protective against asthma.[ref]
On the flip side, in older farmers, the risk of respiratory issues is very high. “Farmer’s Lung” is a term applied to lung problems from exposure to mold spores, dust, and other respiratory irritants.[ref]
Genetics and Asthma:
Early genetic studies showed that asthma is about 60% heritable, which indicates a strong genetic susceptibility combined with lung exposure to viruses, bacteria, and lung irritants such as cigarette smoke.[ref] Genome-wide association studies identified key pathways involved in asthma, and newer studies have refined the genetic susceptibility component of the different types of asthma.
Genetic variants related to inflammatory cytokines, immune response, and inflammation in the lungs have all been identified as increasing susceptibility to asthma.
In the Genotype report section below, the genetic variants related to asthma are broadly grouped according to T2-high, T2-low, histamine, or environmental factors. Please keep in mind that many people will have a combination of genetic susceptibility factors that uniquely interact with their environment.
Genotype report: Asthma genes
Lifehacks: What works for asthma?
Please talk with your doctor about a plan that works best for your condition. Before starting any supplements, be sure to check with your doctor or pharmacist for medication interactions.
General asthma treatments (selected clinical trials):
Albuterol:
Albuterol is a fast-acting medication that relaxes and opens the airways, making it easier to breathe. It acts on β2-adrenergic receptors (beta agonist), which causes bronchial smooth muscle to relax and inhibits the release of mast cell mediators. A small amount of albuterol can be detected in the blood within 2 to 3 hours after inhalation and has a half-life of 4 to 5 hours. The most common adverse effects are tremors and nervousness. Other side effects include insomnia and nausea (1 in 10 patients). [ref]
Corticosteroids and long-acting inhalers:
The current mainstay of asthma medications is a combination of corticosteroids and long-acting bronchodilators. These types of medications reduce asthma exacerbations by 50% in some studies. [ref][ref]
Research shows that people with the T2-high phenotype may respond better to corticosteroids, while the non-eosinophilic types of asthma are less likely to respond to corticosteroids.[ref]
Montelukast:
Montelukast is a leukotriene receptor antagonist approved in 1998 for the treatment of asthma (US). Several clinical trials show that it has no statistical effect on asthma symptoms and severity, while others show that it may help in certain situations (such as in smokers). [ref][ref][ref][ref][ref] The FDA added a black box warning in 2020 for suicidal thoughts.[ref]
Physical interventions:
Alpine altitude:
Alpine altitude climate treatment (AACT) is a European treatment program available since the 19th century for tuberculosis and now for asthma and chronic rhinitis/sinusitis. The clear mountain air and sunshine are believed to be protective and healing. These mountain retreats in the Alps have low pollution, low humidity, low barometric pressure, and low airborne allergies.[ref]
Breathing exercises:
Multiple clinical trials show that “inspiratory muscle training” for 6 to 12 weeks improved asthma symptoms better than conventional breathing exercises.[ref][ref] Inspiratory muscle training uses a device you breathe through that makes inhaling a bit more difficult to force your diaphragm and intercostal muscles to work better.
Specific lifehacks based on genetic pathways:
Low histamine diet (histamine-related genes):
Foods high in histamine can contribute to overall histamine levels in the body. A two-period, randomized, crossover pilot study investigated whether histamine levels in foods affect asthma in children. The results showed that when the participants ate a normal diet high in histamine, they had more airflow obstruction.[ref]
Folate-rich foods or methylfolate (MTHFR variant):
Low folate levels in children are associated with an increased risk of asthma. In adults with asthma, lower folate levels are associated with higher eosinophil levels. Folate is used in the methylation cycle to produce methyl groups, and methyl groups are needed to break down histamine. [ref][ref]
You can increase your folate levels by eating folate-rich foods (leafy greens, legumes, liver) or by taking supplemental methylfolate. Folic acid is the synthetic form of folate, and not everyone converts it as well to folate. Importantly, a study shows that higher levels of methylfolate (active folate) are associated with lower asthma symptoms and better lung function, while higher levels of unmetabolized folic acid in the blood show the opposite.[ref] In addition, high levels of folic acid from prenatal vitamins are associated with an increased risk of asthma in children.[ref][ref]
P5P (active B6) for IL-33:
A recent study found that people with asthma have lower concentrations of P5P, the active form of vitamin B6, and the levels correlate with worsening lung function and higher inflammation. IL-33 is regulated in a P5P-dependent process, and animal and cell line studies show that increasing P5P reduces IL-33 inflammation.[ref] When looking at supplements with P5P (pyridoxal 5′-phosphate), you’ll find that most contain 50 to 100 mg doses, which is at the upper daily limit for vitamin B6 for adults. You may want to consider starting with a lower dose, such as 25mg or less. B6 is a cofactor for many cellular reactions, so starting with a lower dose may be a safer way to go.
Related article: Vitamin B6
Sulforaphane or cruciferous vegetables (GST variants):
Several studies looked at the effects of sulforaphane supplementation on inflammatory lung diseases. Animal studies show that sulforaphane can reverse allergic asthma.[ref] Animal studies also show that the Nrf2 pathway is critical for the prevention of pulmonary fibrosis.[ref] Oral sulforaphane as 200g of broccoli sprouts was shown to increase phase II enzyme expression in the upper airways.[ref] Additionally, a study showed reduced bronchoconstriction, and another showed that it reduced allergic response to diesel exhaust particles.[ref][ref]
Nigella sativa (black seed oil) for Th2-high and Th2-low:
Black seed oil (Nigella sativa) is a natural anti-inflammatory and antimicrobial. Multiple studies show that Nigella sativa extract improves asthma symptoms. Clinical trials also show that nigella sativa decreases eosinophil levels and improves forced expiratory volume. One clinical trial used 1 to 2 g/day of Nigella sativa for three months and saw improvements in lung function and decreases in exacerbations. Animal studies show that Nigella sativa inhibits histamine release. [ref][ref][ref][ref][ref]
Ginkgo biloba for Th2-low or TNF:
A study in asthma patients found that Ginkgo biloba reduced TNF-alpha and other inflammatory cytokine levels and improved pulmonary function (FEV1). Another study looked at the addition of Ginkgo biloba extract to asthma treatment (fluticasone propionate) compared to just fluticasone propionate alone. The results showed decreased IL-5 and decreased infiltration of the airways with eosinophils.[ref][ref]
Boswellia serrata (Frankincense):
Several studies have shown that Boswellia serrata, traditionally used in Ayurveda or Traditional Persian Medicine, improves symptoms of asthma. This is likely due to the antifungal and antiviral effects of frankincense.[ref][ref][ref][ref]
Vitamin C:
Several studies have looked at vitamin C, a dietary antioxidant, as a beneficial supplement for asthma. A Cochrane review of studies found little improvement with vitamin C supplementation for exercise-induced bronchoconstriction. In children, vitamin C plus zinc has a positive effect on asthma symptoms.[ref][ref] However, a Mendelian randomization study showed no causal association between vitamin C levels and adult asthma risk.[ref] And a randomized, placebo-controlled trial of vitamin C plus magnesium in adults found no beneficial effects.[ref]
Related articles: