Key takeaways:
~ TNF-alpha (tumor necrosis factor alpha) is an inflammatory cytokine produced by the immune system to fight off pathogens and cancer cells.
~ Chronic elevation of TNF-alpha levels is linked to autoimmune diseases, skin infections, gum disease, heart disease, depression, and neurodegenerative diseases.
~ Genetic variants can increase your susceptibility to chronically elevated TNF-alpha.
~ Understanding your genes can help you find targeted, natural solutions for chronic inflammation.
Members will see their genotype report below, plus natural solutions for elevated TNF levels in the Lifehacks section. Consider joining today.
What is TNF Alpha?
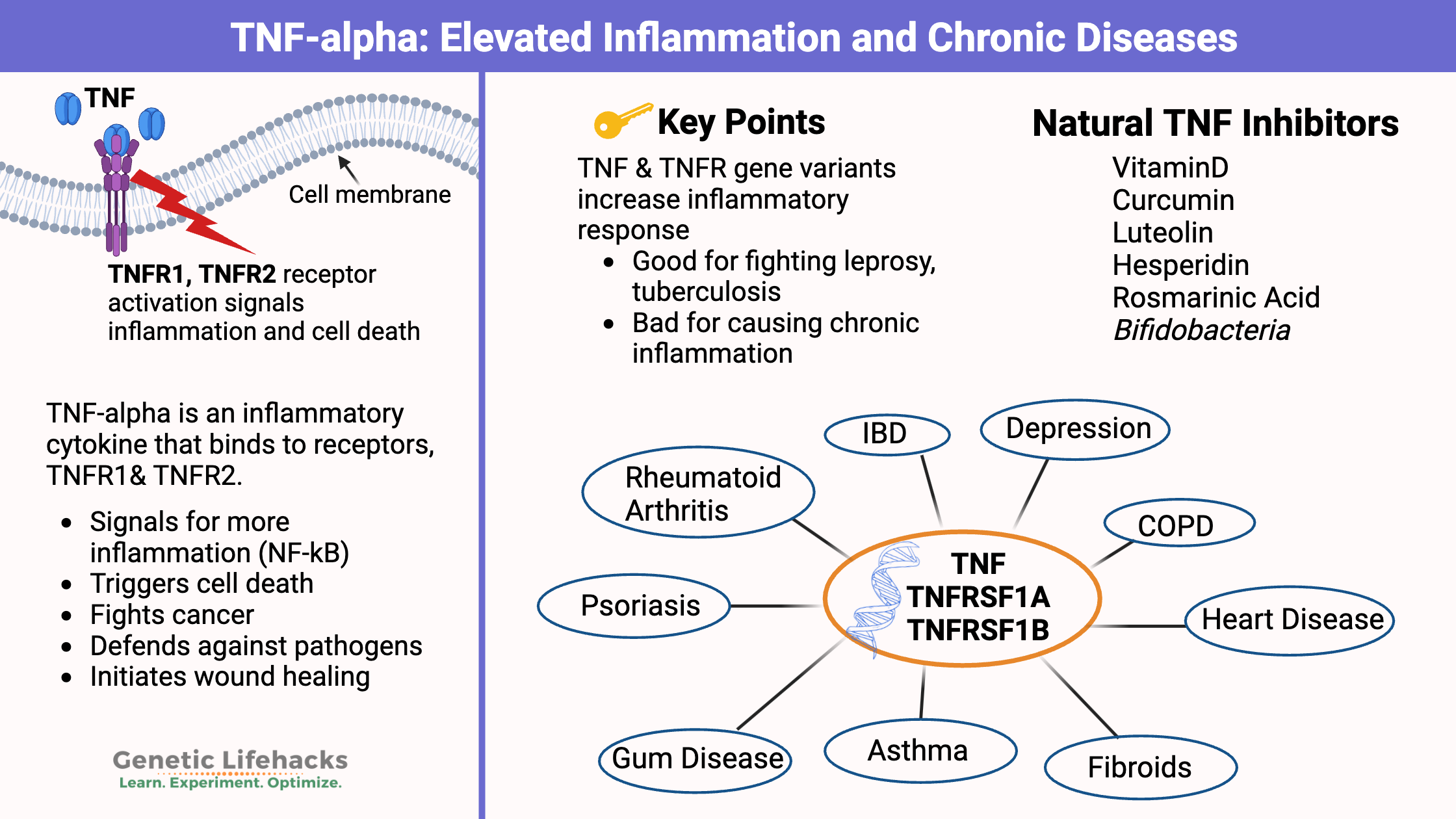
TNF-alpha (tumor necrosis factor alpha) is an inflammatory cytokine produced by certain immune system cells during acute inflammation. The main role of this cytokine involves signaling for ‘apoptosis’ meaning the cell needs to be destroyed. This is important both in fighting off a pathogen as well as in killing cancer cells.[ref]
Inflammatory signaling molecule: Calling in the troops
We think of redness, swelling, heat, and pain as inflammation, such as what happens after getting injured. However, the inflammatory response is also vital in fighting off bad bacteria, viruses, or fungi.
When the body needs an inflammatory response to fight off an invader, it releases inflammatory cytokines, such as TNF-alpha (and others).
When TNF-alpha binds to its receptor on the surface of a cell, two actions can occur:
- One option is that it kills the cell. It’s like it pulls the pin on a grenade.
- Another option is that it can also cause the cell to produce other inflammatory response molecules, but the cell still survives.
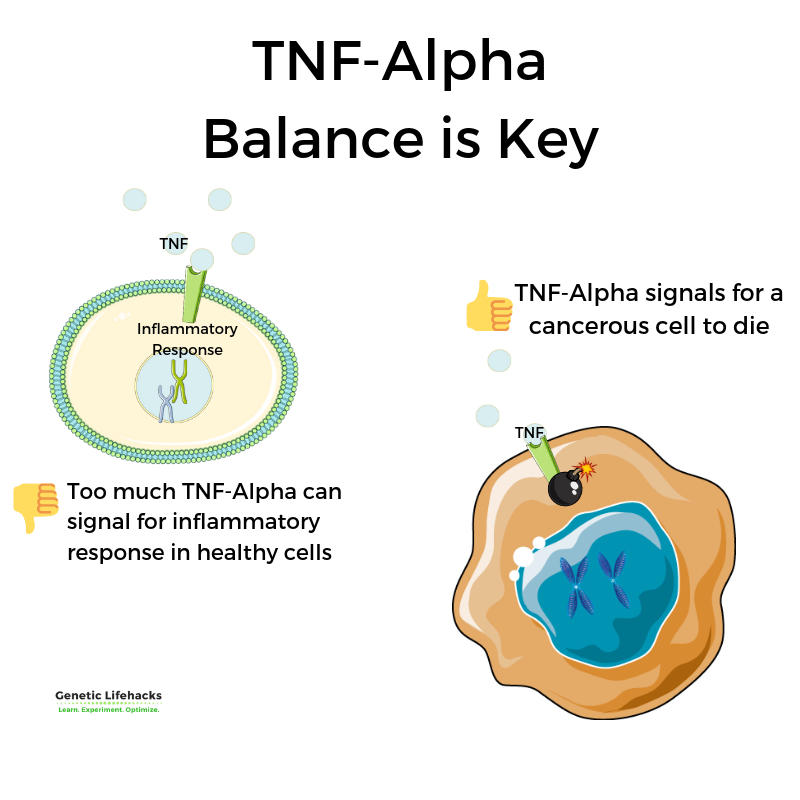
While cell death sounds bad, it is completely necessary to fight off certain infections — or if a cell is cancerous. Thus the “tumor” in tumor necrosis factor (TNF).
Wound healing:
Additionally, TNF-alpha is essential for the initial stages of wound healing. Inflammation is a necessary step for healing injured tissue.[ref]
Pathogen defense:
TNF-alpha is an essential part of the body’s initial defense against pathogens such as:[ref][ref][ref][ref]
- Tuberculosis
- Histoplasmosis
- Malaria
- Leprosy
Tumor Necrosis Factor α: Signal and Receptor
TNF-alpha is synthesized and released by activated immune cells – macrophages, lymphocytes, mast cells, and neutrophils. It is also produced as a transmembrane protein, as well as by smooth muscle cells in response to injury.[ref][ref]
After being released by a cell (e.g. mast cell or macrophage), TNF alpha can bind to several different receptors on the surfaces of cells.
The two main TNF-alpha receptors are TNFR1 and TNFR2. Most cells in the body have the TNFR1 receptor, but the TNFR2 receptor is more specific to immune system cells.[ref]
When TNF binds to a receptor, several different actions can occur, depending on the cell type and receptor type:
- activates NF-κB, which is a transcription factor that controls cell survival and inflammatory response
- activates the MAPK pathways, which are important in the cell cycle and preventing cancer
- signals for cell death
How does TNF alpha cause inflammation?
The immune response is all about balance. You want to fight off pathogens with a strong response, but you don’t want a constant inflammatory response continuing when it isn’t needed.
Super fighter:
Genetic variants that increase TNF-alpha levels are linked to being better able to fight off pathogens, such as malaria or hepatitis B.[ref]
But.. That superpower of fighting pathogens and killing cancer cells comes with a price. For example, chronically elevated levels of TNF-alpha are linked with an increased risk of autoimmune diseases, skin infections, and gum disease.
Chronic inflammation in the body:
Several common genetic variants increase TNF-alpha levels and increase the risk of inflammatory conditions. Keep in mind that these same genetic variants that helped your ancestors survive leprosy or tuberculosis (and thus lived to pass on the variant to you), could be at the root of many of the inflammatory conditions that plague us today.[ref]
Higher TNF-alpha is linked to:
- Rheumatoid arthritis (RA)
- Psoriasis
- IBD (ulcerative colitis, Crohn’s disease)
- Skin infections
- Gum disease
- Asthma
- Diabetic ulcers
- Heart disease
- Septic shock
- Ankylosing spondylitis
- Depression
- Arthritis
- COPD
- Neurodegenerative diseases
Studies relate TNF alpha to chronic diseases:
Chronic inflammation in the body can be a driving factor in many inflammatory-based chronic diseases.
Examples of diseases caused by inflammation include:
- Depression and mood disorders
- Heart disease
- Fibroids
- Neurodegenerative diseases
- Arthritis
- Fatty liver disease (NAFLD)
Let’s dive into the research here:
Inflammation and Depression:
Recent research shows that, for some people, pro-inflammatory cytokines are at the heart of a major depressive disorder. For a subset of patients with depression, TNF alpha is elevated, and blocking TNF-alpha can ameliorate depressive symptoms. A 2008 genome-wide association study found that a TNF genetic variant increases the risk of depression.[ref]
Related article: Depression and Inflammation
Researchers think that inflammation, specifically high TNF alpha levels, impacts the HPA axis and elevates cortisol. HPA axis dysfunction is strongly linked to depression. Additionally, higher TNF-alpha levels can cause increased uptake of serotonin into cells, causing depressive symptoms.[ref]
Related article: HPA axis dysfunction and your genes
Heart disease:
TNF alpha is increased in heart disease, raising the question of whether heart disease causes an increase in TNF-alpha or whether higher TNF-alpha contributes to causing heart disease. To answer this question, researchers turned to genetics. Studies show variants that increase TNF are causally linked to an increased risk of heart disease.[ref]
Related article: Genetics and Coronary Artery Disease
TNF and Uterine fibroids:
Uterine fibroids are benign tumors in the uterus. These are a common occurrence, with estimates ranging from 50-80% of women having fibroids at some point in their lives. The growth of the fibroids is thought to be caused by steroid hormones (estrogen, progesterone), growth factors, cytokines, and chemokines. Research points to TNF-alpha as the most important cytokines involved in fibroid growth.[ref]
Related article: Fibroids genes
Intestinal absorption is altered with high TNF-alpha:
Inflammation and high TNF-alpha levels in the intestines alter the ability to absorb nutrients.
For example:
- High TNF-alpha levels decrease the receptor needed for the absorption of vitamin C (SLC23A1).[ref]
- Short-chain fatty acids, such as butyrate, are important for colon health. High levels of TNF-alpha decrease the expression of transporters for butyrate (SLC5A8).[ref]
- Phosphate absorption is decreased when TNF-alpha is high in the intestines. Low phosphate absorption can cause problems with teeth and bones.[ref]
Neurodegenerative diseases:
Alzheimer’s and Parkinson’s diseases are both linked to higher TNF-alpha production in the brain.
Research shows that microglia and astrocytes release TNF-alpha when activated, and this increases the production of amyloid-beta plaque, which is linked with Alzheimer’s disease. Additionally, amyloid-beta can activate microglia and astrocytes, further perpetuating the creation of TNF-alpha and other inflammatory cytokines.[ref][ref]
Related article: Alzheimer’s APOE genotype
Fatty liver disease:
Non-alcoholic fatty liver disease (NAFLD) is caused by increased fat stored in the liver. This can eventually lead to liver disease. TNF-alpha, as well as a couple of other inflammatory cytokines, are increased in fatty liver disease. Research using animals that were genetically altered to reduce TNF-alpha levels shows that TNF-alpha is a driving factor in fatty liver disease.[ref][ref]
Related article: Fatty liver disease (NAFLD) genes
TNF Genotype Report
Members: Log in to see your data below.
Not a member? Join here.
Why is this section is now only for members? Here’s why…
Lifehacks: Natural ways to inhibit TNF alpha
If you carry genetic variants related to higher TNF-alpha levels and have a related inflammatory condition, inhibiting TNF-alpha may help to reduce chronic inflammation.[ref]
Keep in mind the tradeoff between TNF-alpha as a response to pathogens and inhibiting TNF-alpha to reduce chronic inflammation. If you are at a higher risk for infections, talk to your doctor.
The rest of this article covers natural TNF inhibitors, lifehacks, next steps to take, and how to prioritize the solutions. It is for Genetic Lifehacks members only. Consider joining today to see the rest of this article.
Related Articles and Topics:
BDNF: Optimizing your brain
The BDNF gene encodes brain-derived neurotrophic factor, which is important in neurogenesis, resilience, and introversion.
Phenylalanine and Phenylketonuria
Learn how phenylalanine impacts brain function and which genetic mutations cause phenylketonuria.
Always tired? Genetic reasons for fatigue
Are you always tired even when you know you slept well? Discover more about the newest research on fatigue and how genetic susceptibility plays a part for some people.
CTLA-4: Autoimmune Genetic Risk
The CTLA4 gene codes for a protein that is important in the immune system. It acts as a checkpoint that can downregulate your immune system response. Genetic variants in the CTLA4 gene can increase your risk for several different autoimmune diseases.
Psoriasis Genes
Psoriasis is an autoimmune condition that causes dry, sometimes itchy patches of skin. It is caused by the immune system attacking your skin cells, speeding up the turnover of the cells. Genetics plays a role in your susceptibility
Longevity Lifehacks: TNF-alpha and Inflammaging
Learn more about how chronically elevated TNF-alpha is a cause of chronic disease in aging.
References:
Allendoerfer, Ruth, and George S. Deepe. “Blockade of Endogenous TNF-α Exacerbates Primary and Secondary Pulmonary Histoplasmosis by Differential Mechanisms.” The Journal of Immunology, vol. 160, no. 12, June 1998, pp. 6072–82. www.jimmunol.org, https://www.jimmunol.org/content/160/12/6072.
Borthakur, Alip, et al. “The Probiotic Lactobacillus Plantarum Counteracts TNF-{alpha}-Induced Downregulation of SMCT1 Expression and Function.” American Journal of Physiology. Gastrointestinal and Liver Physiology, vol. 299, no. 4, Oct. 2010, pp. G928-934. PubMed, https://doi.org/10.1152/ajpgi.00279.2010.
Chen, Huacong, et al. “Tumor Necrosis Factor-Alpha Impairs Intestinal Phosphate Absorption in Colitis.” American Journal of Physiology. Gastrointestinal and Liver Physiology, vol. 296, no. 4, Apr. 2009, pp. G775-781. PubMed, https://doi.org/10.1152/ajpgi.90722.2008.
Chen, Yihe, and Kuifen Ma. “NLRC4 Inflammasome Activation Regulated by TNF-α Promotes Inflammatory Responses in Nonalcoholic Fatty Liver Disease.” Biochemical and Biophysical Research Communications, vol. 511, no. 3, Apr. 2019, pp. 524–30. PubMed, https://doi.org/10.1016/j.bbrc.2019.02.099.
Ciebiera, Michał, et al. “The Role of Tumor Necrosis Factor α in the Biology of Uterine Fibroids and the Related Symptoms.” International Journal of Molecular Sciences, vol. 19, no. 12, Dec. 2018, p. 3869. PubMed Central, https://doi.org/10.3390/ijms19123869.
—. “The Role of Tumor Necrosis Factor α in the Biology of Uterine Fibroids and the Related Symptoms.” International Journal of Molecular Sciences, vol. 19, no. 12, Dec. 2018, p. 3869. PubMed Central, https://doi.org/10.3390/ijms19123869.
Culjak, Marija, et al. “The Association between TNF-Alpha, IL-1 Alpha and IL-10 with Alzheimer’s Disease.” Current Alzheimer Research, vol. 17, no. 11, 2020, pp. 972–84. PubMed, https://doi.org/10.2174/1567205017666201130092427.
Dhamodharan, Umapathy, et al. “Genetic Association of IL-6, TNF-α and SDF-1 Polymorphisms with Serum Cytokine Levels in Diabetic Foot Ulcer.” Gene, vol. 565, no. 1, July 2015, pp. 62–67. PubMed, https://doi.org/10.1016/j.gene.2015.03.063.
Ding, Cheng, et al. “TNF-α Gene Promoter Polymorphisms Contribute to Periodontitis Susceptibility: Evidence from 46 Studies.” Journal of Clinical Periodontology, vol. 41, no. 8, Aug. 2014, pp. 748–59. PubMed, https://doi.org/10.1111/jcpe.12279.
Durães, Cecília, et al. “Polymorphisms in the TNFA and IL6 Genes Represent Risk Factors for Autoimmune Thyroid Disease.” PloS One, vol. 9, no. 8, 2014, p. e105492. PubMed, https://doi.org/10.1371/journal.pone.0105492.
—. “Polymorphisms in the TNFA and IL6 Genes Represent Risk Factors for Autoimmune Thyroid Disease.” PloS One, vol. 9, no. 8, 2014, p. e105492. PubMed, https://doi.org/10.1371/journal.pone.0105492.
Feng, Bo, et al. “Association of Tumor Necrosis Factor α -308G/A and Interleukin-6 -174G/C Gene Polymorphism with Pneumonia-Induced Sepsis.” Journal of Critical Care, vol. 30, no. 5, Oct. 2015, pp. 920–23. PubMed, https://doi.org/10.1016/j.jcrc.2015.04.123.
Frankola, Kathryn A., et al. “Targeting TNF-Alpha to Elucidate and Ameliorate Neuroinflammation in Neurodegenerative Diseases.” CNS & Neurological Disorders Drug Targets, vol. 10, no. 3, May 2011, pp. 391–403. PubMed Central, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4663975/.
Gichohi-Wainaina, Wanjiku N., et al. “Tumour Necrosis Factor Allele Variants and Their Association with the Occurrence and Severity of Malaria in African Children: A Longitudinal Study.” Malaria Journal, vol. 14, June 2015, p. 249. PubMed, https://doi.org/10.1186/s12936-015-0767-3.
Idriss, H. T., and J. H. Naismith. “TNF Alpha and the TNF Receptor Superfamily: Structure-Function Relationship(s).” Microscopy Research and Technique, vol. 50, no. 3, Aug. 2000, pp. 184–95. PubMed, https://doi.org/10.1002/1097-0029(20000801)50:3<184::AID-JEMT2>3.0.CO;2-H.
Kakino, Satomi, et al. “Pivotal Role of TNF-α in the Development and Progression of Nonalcoholic Fatty Liver Disease in a Murine Model.” Hormone and Metabolic Research = Hormon- Und Stoffwechselforschung = Hormones Et Metabolisme, vol. 50, no. 1, Jan. 2018, pp. 80–87. PubMed, https://doi.org/10.1055/s-0043-118666.
Khan, Saif, et al. “TNF-α -308 G > A (Rs1800629) Polymorphism Is Associated with Celiac Disease: A Meta-Analysis of 11 Case-Control Studies.” Scientific Reports, vol. 6, Sept. 2016, p. 32677. PubMed, https://doi.org/10.1038/srep32677.
Locksley, Richard M., et al. “The TNF and TNF Receptor Superfamilies: Integrating Mammalian Biology.” Cell, vol. 104, no. 4, Feb. 2001, pp. 487–501. www.cell.com, https://doi.org/10.1016/S0092-8674(01)00237-9.
Ma, Ke, et al. “Pathogenetic and Therapeutic Applications of Tumor Necrosis Factor-α (TNF-α) in Major Depressive Disorder: A Systematic Review.” International Journal of Molecular Sciences, vol. 17, no. 5, May 2016, p. 733. PubMed Central, https://doi.org/10.3390/ijms17050733.
MacIntyre, Elaina A., et al. “GSTP1 and TNF Gene Variants and Associations between Air Pollution and Incident Childhood Asthma: The Traffic, Asthma and Genetics (TAG) Study.” Environmental Health Perspectives, vol. 122, no. 4, Apr. 2014, pp. 418–24. PubMed, https://doi.org/10.1289/ehp.1307459.
Majumder, Poulami, et al. “Association of Tumor Necrosis Factor-α (TNF-α) Gene Promoter Polymorphisms with Aggressive and Chronic Periodontitis in the Eastern Indian Population.” Bioscience Reports, vol. 38, no. 4, Aug. 2018, p. BSR20171212. PubMed, https://doi.org/10.1042/BSR20171212.
Olszewski, Maciej B., et al. “TNF Trafficking to Human Mast Cell Granules: Mature Chain-Dependent Endocytosis.” Journal of Immunology (Baltimore, Md.: 1950), vol. 178, no. 9, May 2007, pp. 5701–09. PubMed, https://doi.org/10.4049/jimmunol.178.9.5701.
“Pathophysiology of Autoimmune Diseases and Inflammation.” AJMC, https://www.ajmc.com/view/pathophysiology-of-autoimmune-diseases-and-inflammation. Accessed 10 Dec. 2021.
Randall, Louise M., and Christian R. Engwerda. “TNF Family Members and Malaria: Old Observations, New Insights and Future Directions.” Experimental Parasitology, vol. 126, no. 3, Nov. 2010, pp. 326–31. ScienceDirect, https://doi.org/10.1016/j.exppara.2010.04.016.
—. “TNF Family Members and Malaria: Old Observations, New Insights and Future Directions.” Experimental Parasitology, vol. 126, no. 3, Nov. 2010, pp. 326–31. ScienceDirect, https://doi.org/10.1016/j.exppara.2010.04.016.
Reséndiz-Hernández, Juan Manuel, et al. “Identification of Genetic Variants in the TNF Promoter Associated with COPD Secondary to Tobacco Smoking and Its Severity.” International Journal of Chronic Obstructive Pulmonary Disease, vol. 10, 2015, pp. 1241–51. PubMed, https://doi.org/10.2147/COPD.S83298.
Stappers, M. H. T., et al. “Polymorphisms in Cytokine Genes IL6, TNF, IL10, IL17A and IFNG Influence Susceptibility to Complicated Skin and Skin Structure Infections.” European Journal of Clinical Microbiology & Infectious Diseases: Official Publication of the European Society of Clinical Microbiology, vol. 33, no. 12, Dec. 2014, pp. 2267–74. PubMed, https://doi.org/10.1007/s10096-014-2201-0.
Subramanian, Veedamali S., et al. “Tumor Necrosis Factor Alpha Reduces Intestinal Vitamin C Uptake: A Role for NF-ΚB-Mediated Signaling.” American Journal of Physiology – Gastrointestinal and Liver Physiology, vol. 315, no. 2, Aug. 2018, pp. G241–48. PubMed Central, https://doi.org/10.1152/ajpgi.00071.2018.
Tavares, M., et al. “Tumour Necrosis Factor-Alpha (-308G/A) Promoter Polymorphism Is Associated with Ulcerative Colitis in Brazilian Patients.” International Journal of Immunogenetics, vol. 43, no. 6, Dec. 2016, pp. 376–82. PubMed, https://doi.org/10.1111/iji.12289.
Ueda, Mayu, et al. “A Short-Term Treatment with Tumor Necrosis Factor-Alpha Enhances Stem Cell Phenotype of Human Dental Pulp Cells.” Stem Cell Research & Therapy, vol. 5, no. 1, Feb. 2014, p. 31. PubMed Central, https://doi.org/10.1186/scrt420.
Wu, Jun-Cang, et al. “Gene Polymorphisms and Circulating Levels of the TNF-Alpha Are Associated with Ischemic Stroke: A Meta-Analysis Based on 19,873 Individuals.” International Immunopharmacology, vol. 75, Oct. 2019, p. 105827. PubMed, https://doi.org/10.1016/j.intimp.2019.105827.
Yucesoy, Berran, et al. “Genetic Variants in TNFα, TGFB1, PTGS1 and PTGS2 Genes Are Associated with Diisocyanate-Induced Asthma.” Journal of Immunotoxicology, vol. 13, no. 1, 2016, pp. 119–26. PubMed, https://doi.org/10.3109/1547691X.2015.1017061.
Zhang, Guimin, et al. “The Role of TNF Alpha Polymorphism and Expression in Susceptibility to Nasal Polyposis.” Immunological Investigations, vol. 47, no. 4, May 2018, pp. 360–71. Taylor and Francis+NEJM, https://doi.org/10.1080/08820139.2018.1433204.
Zhang, Peng, et al. “Tumor Necrosis Factor-Alpha Gene Polymorphisms and Susceptibility to Ischemic Heart Disease.” Medicine, vol. 96, no. 14, Apr. 2017, p. e6569. PubMed Central, https://doi.org/10.1097/MD.0000000000006569.
—. “Tumor Necrosis Factor-Alpha Gene Polymorphisms and Susceptibility to Ischemic Heart Disease.” Medicine, vol. 96, no. 14, Apr. 2017, p. e6569. PubMed Central, https://doi.org/10.1097/MD.0000000000006569.