Key takeaways:
~Mast cells release histamine when triggered.
~Estrogen receptors on mast cells may make them slightly more easily triggered when estrogen levels are high.
~Histamine from certain foods can add to your overall histamine levels.
~Genetic variants impact how well you break down histamine and get rid of histamine.
~ Endocrine disruptors, such as BPA and PFOAs, can also bind to estrogen receptors on mast cells, increasing degranulation.
Members will see their genotype report below, plus many additional solutions in the Lifehacks section. Consider joining today.
Estrogen, Histamine Intolerance, and Mast Cell Activation:
Estrogen is one of those hormones that everyone knows about, but most of us don’t really understand how it works. I know that I didn’t.
Both men and women produce estrogen as a steroid hormone, which is derived from cholesterol.
Women produce more estrogen than men, especially during reproductive years (prior to menopause). In women, estrogen is created in the ovaries in larger amounts. Men convert testosterone into estrogen, and estrogen concentrations are actually the highest in the testes.[ref]
Ok, but what exactly does estrogen do?
Estrogen is a hormone that causes actions in cells to take place by binding to estrogen receptors (ERα, ERβ, and GPER1). Estrogen just by itself isn’t doing much — it is through its binding to the receptors that it initiates different cellular processes.
Estrogen receptors (ERs) are present in a wide variety of tissues in the body. For example, ERs are important in vascular endothelial cells, which line blood vessels. Estrogen receptors are found in cardiomyocytes (heart muscle cells), neurons, airway cells, muscles, the uterus, testes, fat tissue, bone, breast, kidneys, and more.
Estrogen receptors function in two ways:[ref]
- Estrogen receptors (ERs) cause genes in the nucleus to be transcribed (turns on specific estrogen-responsive genes)
- ERs can trigger rapid activation of signaling pathways (immediate changes)
In general, estrogens increase growth by turning on genes for transcription that are related to cellular growth.
Cellular growth is necessary and good – except in cases where it promotes cancer growth.
There are several sub-types of estrogen (estrone (E1), 17β-estradiol (E2), estriol (E3), and estetrol (E4). In this article, when I refer to estrogen, I’ll usually be referring to estradiol (E2), which is the physiologically active form.[ref]
Let’s next look at histamine and mast cells, and then I’ll tie everything together by discussing how estrogen could impact histamine levels in the body.
Histamine and Mast Cells
Histamine is a molecule most commonly associated with causing allergy-like symptoms such as runny nose, hives, watery eyes, and even anaphylaxis. These immediate allergy symptoms are due to large amounts of histamine being released and binding to cellular receptors.
Mast cells are the source of the histamine released in an allergic reaction. They can be activated by allergens and release histamine and other inflammatory mediators, with the goal of protecting us from pathogens and foreign substances. Mast cells are a part of the innate immune response.
There’s more to this story than mast cells releasing histamine due to an allergen.
Histamine also acts as a signaling molecule throughout the body at normal levels. It binds to receptors and initiates cellular actions.
For example, histamine in the stomach binds to H2 receptors to initiate the release of stomach acid from cells lining the stomach. It is triggered as you start to eat (or at the time you usually eat), and the stomach acid helps to break down your food.
There are four different histamine receptors with unique functions. Different histamine receptors are found in different parts of the body:[ref]
H1 receptors:
Found in smooth muscle, endothelial cells (lining the blood vessels), the central nervous system, and mast cells. Activating the H1 receptors causes allergy-type symptoms such as itching, swelling, vasodilation, nose running, and skin reactions. H1 receptors are also important in asthma reactions.
H2 receptors:
Acid is secreted in the stomach when histamine activates the H2 receptors. H2 receptors are also found in the intestinal tract and the walls of blood vessels. Mast cells also have H2 receptors, which, when activated, cause the release of more histamine. In the heart, H2 receptors are important in controlling the rhythm.
H3 receptors:
The central and peripheral nervous systems contain H3 receptors, which act as a feedback loop for histamine levels in the brain. Activating the H3 receptors impacts serotonin, norepinephrine, and acetylcholine release.[ref]
H4 receptors:
The H4 receptors are at the heart of the inflammatory response. H4 receptors are found in the bone marrow, basophils (a type of white blood cell), the thymus, small intestine, spleen, colon, and mast cells.[ref]
Histamine from foods:
In addition to creating histamine in the body (mast cells, other immune cells, in the brain as a neurotransmitter), we also get histamine from certain foods.
Here’s a partial list of high-histamine foods:
- Processed meats (deli meat, sausages, beef jerky, pepperoni)
- Most cheeses (except farmer cheese)
- Fish and seafood that isn’t completely fresh
- Spinach
- Chocolate
- Tomatoes
- Strawberries
- Wine and sake
I find this histamine food list to be the most thorough: Complete list of high histamine foods (pdf).
In addition to absorbing histamine from foods, your gut microbes can also produce histamine.[ref]
Mast cells, histamine, and estrogen
As mentioned above, mast cells release large amounts of histamine when triggered to degranulate.
Mast cells are found throughout the body. However, they are more abundant near tissue regularly exposed to pathogens and allergens — skin, respiratory tract, intestinal tract, and blood vessels.
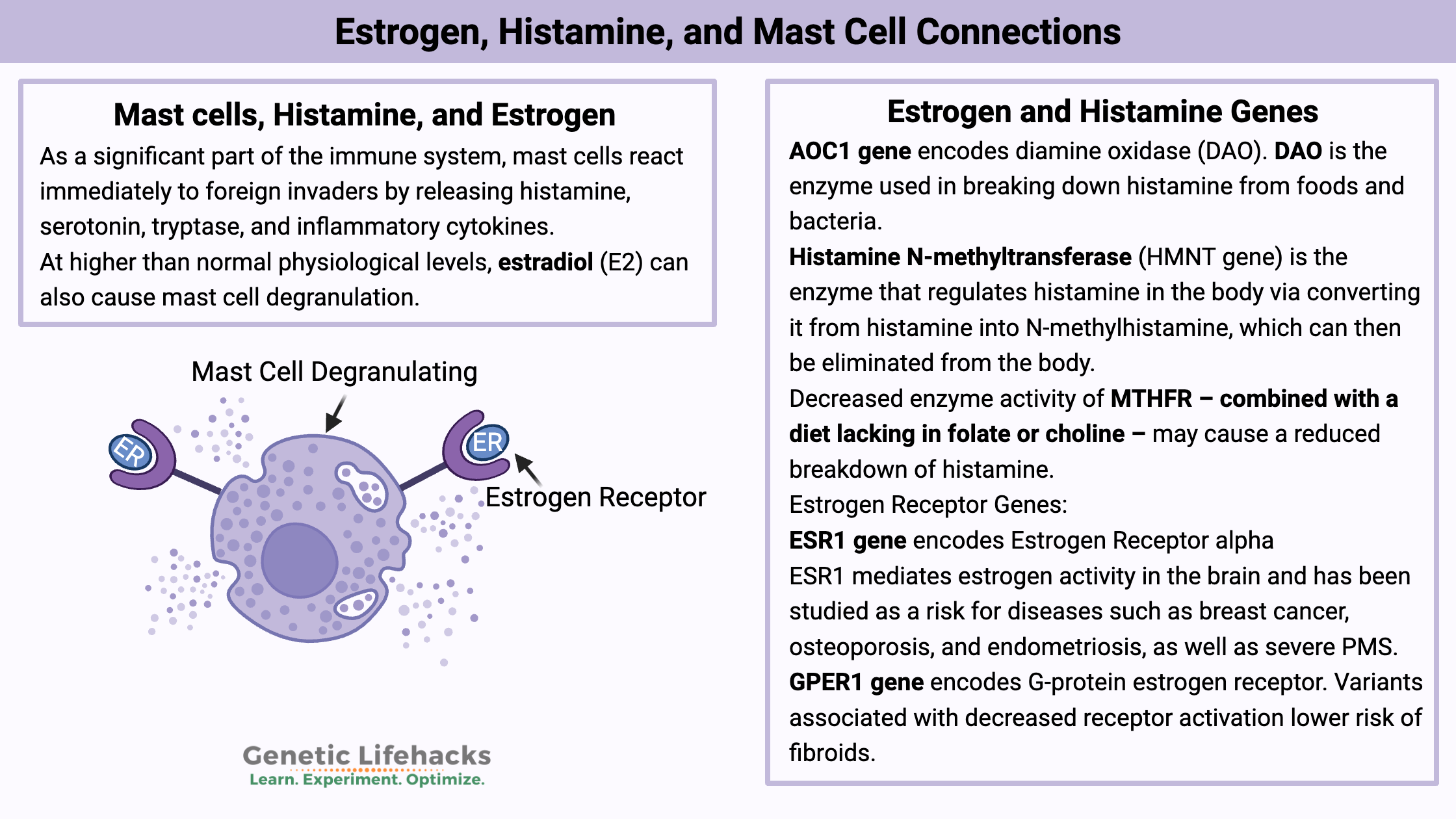
As a significant part of the immune system, mast cells react immediately to foreign invaders by releasing histamine, serotonin, tryptase, and inflammatory cytokines. It is an immediate response to anything perceived as harmful or foreign — essential for reacting quickly to something that shouldn’t be in the body.
The immediate release of mediators from mast cells, such as histamine, can rapidly induce changes, such as to the endothelium lining the blood vessels. The creation and release of inflammatory cytokines can help orchestrate the activity of other immune cells, bringing in T-cells and neutrophils to the area.[ref]
When mast cells are triggered too easily or inadvertently, it can lead to the many different symptoms found in mast cell activation syndrome.
Symptoms of Mast Cell Activation Syndrome:
Symptoms of mast cell activation syndrome can be broken down into the following categories:[ref]
- Skin symptoms: swelling, flushing, itching, hives, dermatographia (skin reaction when scratched)
- Gastrointestinal symptoms: abdominal pain, bloating, diarrhea, heartburn, nausea/vomiting
- Cardiovascular symptoms: chest pain, low blood pressure, altered heart rhythm
- Respiratory symptoms: hoarseness, sore throat, stridor, throat swelling, wheezing
- Neurological symptoms: headache, brain fog, peripheral neuropathy, tingling
- Muscular and bone symptoms: bone or muscle pain, osteoporosis, degenerative disc disease
- Nose and eye symptoms: congestion, itching, and watery eyes
- Systemic symptoms: anaphylaxis, fatigue
People with mast cell activation syndrome usually have some, but not all, of the above symptoms.
How does estrogen interact with mast cells?
Researchers have found that high levels of estradiol can stimulate mast cells to partially degranulate.
At higher than normal physiological levels, estradiol (E2) can also cause mast cell degranulation. The researchers used cell lines and animals to study mast cell degranulation. Essentially, the mast cells were more likely to degranulate when exposed to an allergen if they were also in the presence of estrogen.[ref]
Additional research also points to mast cell activation when estrogen levels increase. For example, mast cell derived leukotrienes (inflammatory mediators) are higher in women right before their periods, when estrogen is highest. Researchers have also shown that medications that block the estrogen receptors, such as tamoxifen, are linked to a decreased release of leukotrienes.[ref]
What this adds up to is that women could be more sensitive to mast cells degranulating in the presence of a triggering compound — especially when estrogen levels fluctuate to higher levels.
Does constant higher estrogen levels, such as in estrogen dominance, always cause mast cell problems? I don’t think the research shows this. When estrogen levels are consistently higher, estrogen receptor alpha is downregulated.[ref]
In other words, higher constant estrogen should cause a decreased production of the estrogen receptor found on mast cells. However, fluctuations in estrogen may cause increased susceptibility to mast cell degranulation. It may be why perimenopausal women are more likely to have issues related to mast cells.[ref]
Endocrine-disrupting chemicals that mimic estrogen:
Added to the estrogen created in the body is exposure to environmental estrogen mimics. BPA is one estrogen mimic that is a component of many types of plastic.
Estrogen-mimicking compounds, such as BPA, can bind to estrogen receptor alpha and cause mast cell degeneration. PFOA and PFOS also bind to estrogen receptor alpha.[ref]
Research in cell lines and in animals shows that environmental estrogens add to the effect of estrogen in the body, causing the activation of mast cells.[ref][ref]
Here’s a good example of how this could be affecting people today…
A 2019 study (in female mice) showed that BPA exposure at normal, human levels had an effect on mast cells in the heart — when exposed to a virus. The study used mice housed in plastic cages and drinking from plastic water bottles and tracked their response to myocarditis (heart inflammation) from a virus. The researchers compared this to a control group of mice with no BPA exposure (glass cage, glass water bottles). The group exposed to BPA in their water and cages had increased myocarditis and pericarditis (25% vs. 10%) when injected with a virus compared to the control group without BPA exposure.[ref]
The BPA exposed group had greater numbers of mast cells in the heart and more degranulation, worsening inflammation, and cardiac fibrosis. Additionally, the BPA exposed group had higher levels of several inflammatory cytokines in the heart.
What does this have to do with mast cells and estrogen? The researchers found that the BPA (again, at normal human exposure levels) increased the number of mast cells in the heart and that the mast cells were more likely to degranulate. From the study: “BPA exposure in drinking water increased mast cell degranulation in the heart overall”.[ref]
Related article: Spike protein, mast cells, histamine, and heart rhythm
Histamine as a mast cell degranulation trigger:
In addition to being triggered by pathogens (bacteria, parasites, viruses) and allergens, high levels of histamine can also cause mast cells to degranulate — giving off even more histamine. This feed-forward loop increases mast cell degranulation in a tissue, such as when your body fights off bacteria in a cut on your skin.
Mast cells have both H1 and H4 histamine receptors on their cell surface. The histamine release from mast cells can trigger nearby mast cells to release more histamine. It also helps to recruit other immune system cell types to the tissue.[ref]
~Mast cells that are triggered release histamine.
~Histamine can also come from foods or your gut microbiome.
~Estrogen receptors are found on mast cells. Increased estrogen may allow mast cells to be more easily triggered.
~Estrogen-mimicking chemicals can bind to estrogen receptors, including on mast cells.
~Histamine can also cause mast cells to degranulate.
Let’s move on to how estrogen interacts with histamine, whether from mast cells or other sources.
Estrogen and histamine in the brain
In the 1970s, researchers confirmed that histamine acts as a neurotransmitter in the brain. Histaminergic neurons have histamine receptors that respond to histamine signaling.[ref]
All four types of histamine receptors, H1R, H2R, H3R, and H4R, are found in the brain. Acting as a neurotransmitter, histamine regulates memory, cognition, emotions, appetite, and sleep. When histamine binds to H1R or H2R receptors, it leads to excitation in the brain, but H3 receptors act to inhibit histaminergic neurons.[ref]
An animal study elucidated that histaminergic neurons were found in the hypothalamus. The study showed that estrogen stimulation affected the level of H1 receptors in the hypothalamus, which is involved in sexual arousal.[ref]
Histamine in the hypothalamus can decrease appetite via certain receptors. Estrogen increases histamine in the brain and thus slightly reduces appetite. Menopause – a lowering of estrogen levels – triggers weight gain, and some researchers think that reduced histamine in the brain could be at play here.[ref]
Migraines and Mast Cells:
Mast cells are also found in the brain. The dura is the thick layer of connective tissue surrounding the brain and spinal cord. The dural layer has nerves that relay pain as well as mast cells.
This connection of nociceptors (pain sensors) and mast cells is thought to play a role in headaches and migraines.
Animal studies show that mast cell degranulation in the dura causes pain similar to migraines. The researchers also found that estradiol increases the number of mast cells in the dura matter.
The authors of the study concluded that the reason women get migraines, especially hormone-related migraines, is likely due to the combination of increased mast cells in the dura combined with exposure to a mast cell degranulation trigger. The degranulation then increases histamine, serotonin, and proteases – creating an inflammatory response in the dura layer and triggering the pain receptors around the brain.[ref]
Histamine and Vasodilation
One role of histamine released from mast cells is to cause blood vessels to dilate (vasodilation). It allows more blood flow into the area of mast cell activation, which is important when fighting a pathogen or foreign substance in, for example, a wound on your skin.
A study on aerobic exercise found that histamine is released locally in the skeletal muscles and acts on H1 and H2 receptors to cause vasodilation. The post-exercise histamine comes from both mast cell degranulation and from the endogenous formation of histamine by histidine decarboxylase.[ref]
While acting through different mechanisms, both estrogen and histamine are vasodilators.
Researchers think estrogen protects premenopausal women from vascular disease and atherosclerosis by inhibiting inflammation and acting as a vasodilator in blood vessels.[ref]
Endothelial nitric oxide also causes blood vessel dilation. Estrogen interacts with estrogen receptors in the heart and endothelium to increase endothelial nitric oxide. Animal studies show that estrogen in female animals promotes systemic anaphylaxis via increased vasodilation with eNOS, which combines with the increased vascular permeability from histamine to intensify the anaphylactic response.[ref][ref]
Asthma, estrogen, and histamine
Estrogen receptors interact with histamine in the smooth muscles of the airway in people with asthma. Interestingly, in children, boys have twice the risk of asthma compared to girls. But in adults, women of reproductive age are more likely to have asthma compared to men. Some theorize that the higher estrogen levels in women contribute to the increased asthma risk.
So — what does estrogen have to do with asthma?
Asthma is caused by an over-reaction in the smooth muscles of the airway. Intracellular calcium [Ca2+ ] levels regulate bronchoconstriction (constricted lungs, wheezing) or bronchodilation (relaxed lungs).
A recent research study found that estrogens reduce calcium response to drugs that cause bronchoconstriction via an estrogen receptor (ERα). The researchers found increased estrogen receptors in the airway smooth muscle cells in people with asthma. In airway cells, exposure to a drug that binds to estrogen receptors increases the response to histamine.[ref]
What does all this mean? Estrogen receptors and estrogen may (slightly) increase the response to histamine, causing an over-activation of the muscles surrounding the lungs. It could be interpreted to mean that high histamine levels plus high estrogen levels increase the likelihood of asthma – or just that can’t breathe right, slightly panicky feeling.
Additionally, estrogen-mimicking compounds can increase the interaction with estrogen receptors, histamine, and asthma. In a study of over 500 teens, girls with higher PFOS levels were more likely to have problems with asthma.[ref]
Estrogen, histamine, anaphylaxis:
In mice, “Anaphylactic responses were more pronounced in female than male mice. The enhanced severity of anaphylaxis in female mice was eliminated after pretreatment with an estrogen receptor antagonist or ovariectomy but restored after administration of estradiol in ovariectomized mice, demonstrating that the sex-specific differences are due to the female steroid estradiol. Estrogen did not affect mast cell responsiveness or anaphylaxis onset. Instead, it increased tissue expression of endothelial nitric oxide synthase (eNOS). Blockage of NOS activity with the inhibitor L-NG-nitroarginine methyl ester or genetic eNOS deficiency abolished the sex-related differences.”
Conclusion: “Our study defines a contribution of estrogen through its regulation of eNOS expression and nitric oxide production to vascular hyperpermeability and intensified anaphylactic responses in female mice, providing additional mechanistic insights into risk factors and possible implications for clinical management in the further exploration of human anaphylaxis.”[ref]
Endometriosis and Mast Cells
Endometriosis occurs when endometrium-like tissue grows outside of the uterus. It is estrogen-dependent.
Mast cells are found in higher than normal numbers in endometriosis tissue. Estrogen, especially when in the presence of IgE (allergen provoked), enhances mast cell activation and is thought to play a role in the development of endometriosis.[ref]
NLRP3 is a receptor that senses danger signals or foreign particles and then calls up a strong inflammatory response — it activates the inflammasome. Recent research brings together estrogen, NLRP3 inflammasome signaling, and mast cells in endometriosis. Researchers discovered that estrogen can increase the expression of NLRP3 via estrogen receptor alpha in mast cells. Estrogen increases the transcription of inflammation-related genes.[ref]
Does estrogen decrease diamine oxidase?
Diamine oxidase (DAO) is the enzyme that is produced in intestinal cells to break down histamine from foods and from gut bacteria.
I have read in several articles online that estrogen decreases diamine oxidase. One article linked to a reference from a rat study in 1986. Other than a few animal studies from decades ago, I’m not finding any research that shows that estrogen impacts diamine oxidase levels. A study published in 2000 showed that estradiol didn’t affect DAO levels in rat kidney cells.[ref]
Estrogen and Histamine Genotype Report
The genetic variants included here impact histamine breakdown and also estrogen receptors. Please note that there is no direct research showing a relationship here between estrogen receptor genes and histamine-related genes. Instead, this is offered to help you determine if genetic variants are adding to your histamine-related issues.
Lifehacks
This section outlines ideas on reducing or balancing estrogen levels, histamine-reducing supplements, and avoiding mast cell triggers.
Balancing estrogen levels:
Eat your broccoli (cruciferous vegetables):
Upping your intake of cruciferous vegetables may help to upregulate some of the enzymes involved in metabolizing estrogen. Cruciferous vegetables that are low in histamine include cabbage, broccoli, and bok choi. Brussels sprouts and chard may be liberators of histamine (see how you do with them). Sauerkraut is usually very high in histamines.
DIM (diindole methane):
DIM is a supplement derived from cruciferous vegetables. A small clinical trial found that DIM (108 mg/day) increases the metabolism of estrogen into the ‘good’ metabolites.[ref] Estrogen can take several paths for metabolism, and some of the ‘bad’ metabolites are linked to an increased relative risk of breast cancer.
Related article: Estrogen metabolism and genetics
Calcium D-glucarate:
Related Articles and Topics:
Brain Fog: Genetics and Solutions
There are multiple causes of the cognitive issues known as ‘brain fog’. Genetics may help you figure out your root cause and personalized solutions.
Mast cell activation syndrome
Take a deep dive into how mast cells work and what goes wrong in mast cell activation syndrome (MCAS).
Estrogen: How it is made and how we get rid of it
Estrogen-from how much is made to how it is broken down – depends on genetics and lifestyle factors and affects both men and women.
MRGPRX2:
Mast cell receptor that has recently been discovered to be responsible for hypersensitivity reactions to drugs.
References:
Anvari, Sara, et al. “Genetic Variation along the Histamine Pathway in Children with Allergic versus Nonallergic Asthma.” American Journal of Respiratory Cell and Molecular Biology, vol. 53, no. 6, Dec. 2015, pp. 802–09. PubMed Central, https://doi.org/10.1165/rcmb.2014-0493OC.
Ayuso, Pedro, et al. “Genetic Variability of Human Diamine Oxidase: Occurrence of Three Nonsynonymous Polymorphisms and Study of Their Effect on Serum Enzyme Activity.” Pharmacogenetics and Genomics, vol. 17, no. 9, Sept. 2007, pp. 687–93. PubMed, https://doi.org/10.1097/FPC.0b013e328012b8e4.
“Best 15 Vitamin B-6 Foods: Benefits and Recipes.” Healthline, 26 May 2017, https://www.healthline.com/health/vitamin-b6-foods.
Boes, Tanner, and Dan Levy. “Influence of Sex, Estrous Cycle and Estrogen on Intracranial Dural Mast Cells.” Cephalalgia : An International Journal of Headache, vol. 32, no. 12, Sept. 2012, pp. 924–31. PubMed Central, https://doi.org/10.1177/0333102412454947.
Bruno, Katelyn Ann, et al. “BPA Alters Estrogen Receptor Expression in the Heart After Viral Infection Activating Cardiac Mast Cells and T Cells Leading to Perimyocarditis and Fibrosis.” Frontiers in Endocrinology, vol. 10, Sept. 2019, p. 598. PubMed Central, https://doi.org/10.3389/fendo.2019.00598.
Cao, X. L., et al. “Concentrations of Bisphenol A in the Composite Food Samples from the 2008 Canadian Total Diet Study in Quebec City and Dietary Intake Estimates.” Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, vol. 28, no. 6, June 2011, pp. 791–98. PubMed Central, https://doi.org/10.1080/19440049.2010.513015.
Cooke, Paul S., et al. “Estrogens in Male Physiology.” Physiological Reviews, vol. 97, no. 3, July 2017, pp. 995–1043. journals.physiology.org (Atypon), https://doi.org/10.1152/physrev.00018.2016.
Dalessandri, Kathie M., et al. “Pilot Study: Effect of 3,3’-Diindolylmethane Supplements on Urinary Hormone Metabolites in Postmenopausal Women with a History of Early-Stage Breast Cancer.” Nutrition and Cancer, vol. 50, no. 2, 2004, pp. 161–67. PubMed, https://doi.org/10.1207/s15327914nc5002_5.
ELDAFIRA, Eldafira, et al. “Polymorphisms of Estrogen Receptor-α and Estrogen Receptor-β Genes and Its Expression in Endometriosis.” Turkish Journal of Pharmaceutical Sciences, vol. 18, no. 1, Feb. 2021, pp. 91–95. PubMed Central, https://doi.org/10.4274/tjps.galenos.2019.94914.
García-Martín, Elena, et al. “Histamine Pharmacogenomics.” Pharmacogenomics, vol. 10, no. 5, May 2009, pp. 867–83. PubMed, https://doi.org/10.2217/pgs.09.26.
Gotoh, Koro, et al. “Hypothalamic Neuronal Histamine Signaling in the Estrogen Deficiency-Induced Obesity.” Journal of Neurochemistry, vol. 110, no. 6, Sept. 2009, pp. 1796–805. PubMed, https://doi.org/10.1111/j.1471-4159.2009.06272.x.
Guo, Xinyue, et al. “NLRP3 Inflammasome Activation of Mast Cells by Estrogen via the Nuclear-Initiated Signaling Pathway Contributes to the Development of Endometriosis.” Frontiers in Immunology, vol. 12, Sept. 2021, p. 749979. PubMed Central, https://doi.org/10.3389/fimmu.2021.749979.
Hon, Yuen Yi, et al. “Endogenous Histamine and Cortisol Levels in Subjects with Different Histamine N-Methyltransferase C314T Genotypes.” Molecular Diagnosis & Therapy, vol. 10, no. 2, 2006, pp. 109–14. PubMed Central, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4178529/.
—. “Endogenous Histamine and Cortisol Levels in Subjects with Different Histamine N-Methyltransferase C314T Genotypes : A Pilot Study.” Molecular Diagnosis & Therapy, vol. 10, no. 2, 2006, pp. 109–14. PubMed, https://doi.org/10.1007/BF03256450.
Hox, Valerie, et al. “Estrogen Increases the Severity of Anaphylaxis in Female Mice through Enhanced Endothelial Nitric Oxide Synthase Expression and Nitric Oxide Production.” The Journal of Allergy and Clinical Immunology, vol. 135, no. 3, Mar. 2015, pp. 729-736.e5. PubMed, https://doi.org/10.1016/j.jaci.2014.11.003.
Hussain, Yasin, et al. “G-Protein Estrogen Receptor as a Regulator of Low-Density Lipoprotein Cholesterol Metabolism: Cellular and Population Genetic Studies.” Arteriosclerosis, Thrombosis, and Vascular Biology, vol. 35, no. 1, Jan. 2015, pp. 213–21. PubMed, https://doi.org/10.1161/ATVBAHA.114.304326.
Jones, Bridgette L., et al. “Genetic Variation in the Histamine Production, Response, and Degradation Pathway Is Associated with Histamine Pharmacodynamic Response in Children with Asthma.” Frontiers in Pharmacology, vol. 7, 2016. www.ncbi.nlm.nih.gov, https://doi.org/10.3389/fphar.2016.00524.
Jotova, I., et al. “Effects of Testosterone and 17, Beta-Estradiol on the Polyamine Metabolism in Cultivated Normal Rat Kidney Epithelial Cells.” Amino Acids, vol. 18, no. 4, 2000, pp. 353–61. PubMed, https://doi.org/10.1007/pl00010322.
Kasap, Burcu, et al. “G-Protein-Coupled Estrogen Receptor-30 Gene Polymorphisms Are Associated with Uterine Leiomyoma Risk.” Bosnian Journal of Basic Medical Sciences, vol. 16, no. 1, Feb. 2016, pp. 39–45. PubMed Central, https://doi.org/10.17305/bjbms.2016.683.
Kempuraj, D., et al. “Luteolin Inhibits Myelin Basic Protein-Induced Human Mast Cell Activation and Mast Cell-Dependent Stimulation of Jurkat T Cells.” British Journal of Pharmacology, vol. 155, no. 7, Dec. 2008, pp. 1076–84. PubMed Central, https://doi.org/10.1038/bjp.2008.356.
Laidlaw, Maggie, et al. “Effects of A Breast-Health Herbal Formula Supplement on Estrogen Metabolism in Pre- and Post-Menopausal Women Not Taking Hormonal Contraceptives or Supplements: A Randomized Controlled Trial.” Breast Cancer : Basic and Clinical Research, vol. 4, Dec. 2010, pp. 85–95. PubMed Central, https://doi.org/10.4137/BCBCR.S6505.
Leurs, R., et al. “H1-Antihistamines: Inverse Agonism, Anti-Inflammatory Actions and Cardiac Effects.” Clinical and Experimental Allergy: Journal of the British Society for Allergy and Clinical Immunology, vol. 32, no. 4, Apr. 2002, pp. 489–98. PubMed, https://doi.org/10.1046/j.0954-7894.2002.01314.x.
Mahmood, Danish. “Histamine H3 Receptors and Its Antagonism as a Novel Mechanism for Antipsychotic Effect: A Current Preclinical & Clinical Perspective.” International Journal of Health Sciences, vol. 10, no. 4, Oct. 2016, pp. 564–75. PubMed Central, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5085352/.
Maintz, L., et al. “Association of Single Nucleotide Polymorphisms in the Diamine Oxidase Gene with Diamine Oxidase Serum Activities.” Allergy, vol. 66, no. 7, July 2011, pp. 893–902. PubMed, https://doi.org/10.1111/j.1398-9995.2011.02548.x.
Meyer, Matthias R., et al. “Non-Genomic Regulation of Vascular Cell Function and Growth by Estrogen.” Molecular and Cellular Endocrinology, vol. 308, no. 1–2, Sept. 2009, pp. 9–16. PubMed Central, https://doi.org/10.1016/j.mce.2009.03.009.
Morgan, Marsha K., et al. “Distribution, Variability, and Predictors of Urinary Bisphenol A Levels in 50 North Carolina Adults over a Six-Week Monitoring Period.” Environment International, vol. 112, Mar. 2018, pp. 85–99. PubMed Central, https://doi.org/10.1016/j.envint.2017.12.014.
Mori, Hiroko, et al. “Estrogenic Regulation of Histamine Receptor Subtype H1 Expression in the Ventromedial Nucleus of the Hypothalamus in Female Rats.” PLoS ONE, vol. 9, no. 5, May 2014, p. e96232. PubMed Central, https://doi.org/10.1371/journal.pone.0096232.
Narita, Shin-ichiro, et al. “Environmental Estrogens Induce Mast Cell Degranulation and Enhance IgE-Mediated Release of Allergic Mediators.” Environmental Health Perspectives, vol. 115, no. 1, Jan. 2007, pp. 48–52. PubMed Central, https://doi.org/10.1289/ehp.9378.
Nesheim, Nils, et al. “Elevated Seminal Plasma Estradiol and Epigenetic Inactivation of ESR1 and ESR2 Is Associated with CP/CPPS.” Oncotarget, vol. 9, no. 28, Apr. 2018, pp. 19623–39. PubMed, https://doi.org/10.18632/oncotarget.24714.
O’Brien, Edmund, et al. “Perinatal Bisphenol A Exposures Increase Production of Pro-Inflammatory Mediators in Bone Marrow-Derived Mast Cells of Adult Mice.” Journal of Immunotoxicology, vol. 11, no. 3, Sept. 2014, pp. 205–12. PubMed, https://doi.org/10.3109/1547691X.2013.822036.
Pakharenko, L. “Effect of Estrogen Receptor Gene ESR1 Polymorphism on Development of Premenstrual Syndrome.” Georgian Medical News, no. 235, Oct. 2014, pp. 37–41.
Perfluorooctanoic Acid (PFOA), Perfluorooctane Sulfonate (PFOS), and Related Chemicals. https://www.cancer.org/healthy/cancer-causes/chemicals/teflon-and-perfluorooctanoic-acid-pfoa.html. Accessed 13 Oct. 2022.
Raje, Nikita, et al. “Genetic Variation within the Histamine Pathway among Patients with Asthma.” The Journal of Asthma : Official Journal of the Association for the Care of Asthma, vol. 52, no. 4, May 2015, pp. 353–62. PubMed Central, https://doi.org/10.3109/02770903.2014.973501.
Romero, Steven A., et al. “Mast Cell Degranulation and de Novo Histamine Formation Contribute to Sustained Postexercise Vasodilation in Humans.” Journal of Applied Physiology, vol. 122, no. 3, Mar. 2017, pp. 603–10. PubMed Central, https://doi.org/10.1152/japplphysiol.00633.2016.
Sánchez-Pérez, Sònia, et al. “Intestinal Dysbiosis in Patients with Histamine Intolerance.” Nutrients, vol. 14, no. 9, Apr. 2022, p. 1774. PubMed, https://doi.org/10.3390/nu14091774.
Shulpekova, Yulia O., et al. “Food Intolerance: The Role of Histamine.” Nutrients, vol. 13, no. 9, Sept. 2021, p. 3207. PubMed Central, https://doi.org/10.3390/nu13093207.
Simon, Tünde, et al. “Asthma Endophenotypes and Polymorphisms in the Histamine Receptor HRH4 Gene.” International Archives of Allergy and Immunology, vol. 159, no. 2, 2012, pp. 109–20. PubMed, https://doi.org/10.1159/000335919.
Son, Jee Hee, et al. “A Histamine-Free Diet Is Helpful for Treatment of Adult Patients with Chronic Spontaneous Urticaria.” Annals of Dermatology, vol. 30, no. 2, Apr. 2018, pp. 164–72. PubMed Central, https://doi.org/10.5021/ad.2018.30.2.164.
Stevenson, Jim, et al. “The Role of Histamine Degradation Gene Polymorphisms in Moderating the Effects of Food Additives on Children’s ADHD Symptoms.” American Journal of Psychiatry, vol. 167, no. 9, Sept. 2010, pp. 1108–15. ajp.psychiatryonline.org (Atypon), https://doi.org/10.1176/appi.ajp.2010.09101529.
Sundermann, Erin E., et al. “A Review of Estrogen Receptor α Gene (ESR1) Polymorphisms, Mood, and Cognition.” Menopause (New York, N.Y.), vol. 17, no. 4, July 2010, pp. 874–86. PubMed Central, https://doi.org/10.1097/gme.0b013e3181df4a19.
Thangam, Elden Berla, et al. “The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets.” Frontiers in Immunology, vol. 9, 2018. Frontiers, https://www.frontiersin.org/articles/10.3389/fimmu.2018.01873.
Theoharides, Theoharis C., et al. “Recent Advances in Our Understanding of Mast Cell Activation – or Should It Be Mast Cell Mediator Disorders?” Expert Review of Clinical Immunology, vol. 15, no. 6, June 2019, pp. 639–56. PubMed Central, https://doi.org/10.1080/1744666X.2019.1596800.
Threlfell, Sarah, et al. “Histamine H3 Receptors Inhibit Serotonin Release in Substantia Nigra Pars Reticulata.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, vol. 24, no. 40, Oct. 2004, pp. 8704–10. PubMed, https://doi.org/10.1523/JNEUROSCI.2690-04.2004.
Yoshikawa, Takeo, et al. “Histamine N-Methyltransferase in the Brain.” International Journal of Molecular Sciences, vol. 20, no. 3, Feb. 2019, p. 737. PubMed Central, https://doi.org/10.3390/ijms20030737.
Zaitsu, Masafumi, et al. “Estradiol Activates Mast Cells via a Non-Genomic Estrogen Receptor-α and Calcium Influx.” Molecular Immunology, vol. 44, no. 8, Mar. 2007, pp. 1977–85. PubMed Central, https://doi.org/10.1016/j.molimm.2006.09.030.
Zhang, Erkang, et al. “Apigenin Inhibits Histamine-Induced Cervical Cancer Tumor Growth by Regulating Estrogen Receptor Expression.” Molecules, vol. 25, no. 8, Apr. 2020, p. 1960. PubMed Central, https://doi.org/10.3390/molecules25081960.
Zhou, Yang, et al. “Interaction Effects of Polyfluoroalkyl Substances and Sex Steroid Hormones on Asthma among Children.” Scientific Reports, vol. 7, Apr. 2017, p. 899. PubMed Central, https://doi.org/10.1038/s41598-017-01140-5.
Zierau, Oliver, et al. “Role of Female Sex Hormones, Estradiol and Progesterone, in Mast Cell Behavior.” Frontiers in Immunology, vol. 3, 2012, p. 169. PubMed, https://doi.org/10.3389/fimmu.2012.00169.
.