Key takeaways:
~Tryptophan is an essential amino acid that influences your mood, sleep, neurotransmitters, and immune response.
~ Tryptophan is converted into serotonin, melatonin, quinolinic acid (neurotoxin), and niacin.
~Learn how tryptophan is absorbed, converted, and utilized by the body.
~Tryptophan metabolism may be important in Long Covid symptoms.
Your genetic variants impact how you utilize tryptophan. In the genotype report section, you will see if you are more likely to convert tryptophan into kynurenine or serotonin. I’ll also explain the implications of increased kynurenine metabolites. Members will see their genotype report below, plus additional solutions in the Lifehacks section. Join today.
What is tryptophan and why do we need it?
Tryptophan is an essential amino acid. The term ‘essential’ here means your body cannot make tryptophan; thus, you must obtain it through your food. Many protein-rich foods, such as dairy, fish, meat, nuts, and seeds, contain tryptophan.
Absorbing tryptophan: When you eat foods that contain tryptophan, the amino acid can be absorbed in the intestines – or – it can be used by bacteria in your gut microbiome.
The absorption of tryptophan in the intestines uses amino acid transporters. Some of the tryptophan you eat is absorbed, and some of it is used by bacteria in your gut. Your gut microbes can use tryptophan to produce serotonin, which then can be absorbed in the intestines.[ref]
Once you absorb tryptophan, your body can use it in several different ways.[ref]
- Kynurenine pathway: The majority of tryptophan is converted into kynurenine. This can eventually lead to tryptophan being converted into niacin. But there are lots of steps along the way and intermediate molecules with a variety of implications for mental health.
- Serotonin pathway: A little bit of the tryptophan you consume is used to make serotonin, which is a neurotransmitter in the brain and in the intestines. Serotonin is the precursor for melatonin, so tryptophan eventually can become melatonin.
- Protein synthesis: While we often focus on neurotransmitter production or kynurenine, tryptophan is also used as an amino acid in synthesizing other proteins in the body. Amino acids are the building blocks for synthesizing proteins, and many proteins created in the body incorporate tryptophan. [ref]
Here’s a quick visual overview of the routes that tryptophan can take.
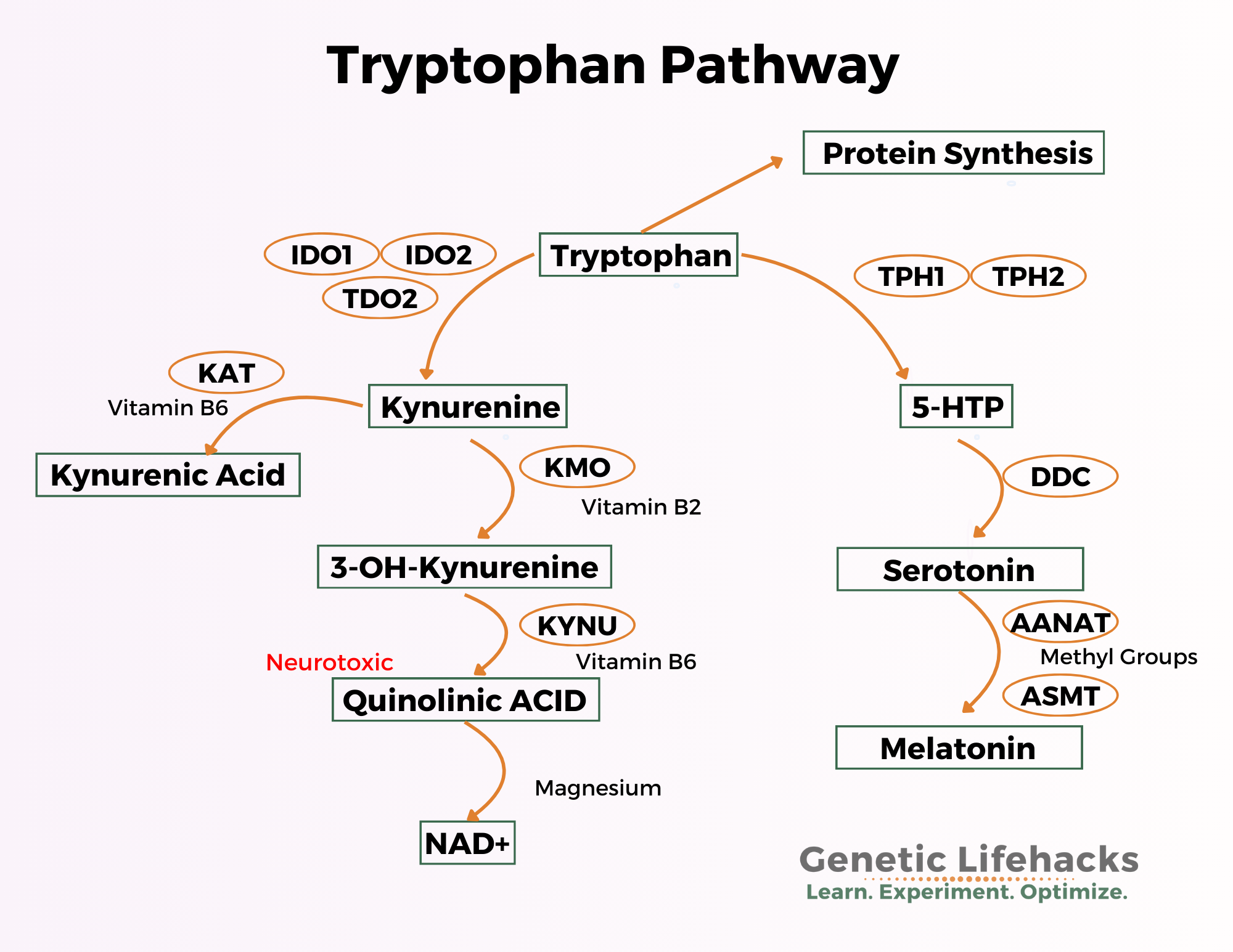
Let’s dive into these pathways, and find out how this impacts neurotransmitters, mood, and immune function. We will start with the left side of the above image – the Kynurenine Pathway.
Kynurenine Pathway:
Around 90-95% of tryptophan is converted into kynurenine.
The conversion of tryptophan to kynurenine needs the IDO enzymes or the TDO enzymes. These enzymes act as catalysts to cause the conversion reaction to occur. [ref]
During normal healthy conditions, tryptophan is converted to kynurenine by the TDO enzyme (tryptophan 2,3 dioxygenase). This enzyme is mainly expressed in the liver and is induced by cortisol and steroid hormones.[ref]
The IDO enzymes (indoleamine 2,3-dioxygenase) can also convert trytophan to kynurenine. They are induced by inflammatory cytokines, such as interferon-gamma and TNF-alpha.
Thus, inflammation may cause tryptophan to be used even more for kynurenine and less for serotonin.
There are two IDO enzymes, IDO1 and IDO2.
- IDO1 is the primary enzyme. It helps to convert tryptophan to kynurenine when the concentration of trytophan is below a certain level (>200 μM). At higher tryptophan concentrations, IDO1 is inhibited through a feedback loop.
- IDO2 is considered to be evolutionarily redundant, and some researchers think that it acts like a fail-safe enzyme that can work at higher tryptophan concentrations. Other research points to it being important for self-tolerance and playing a role in autoimmune diseases and cancer.[ref][ref]
Therefore, when you are stressed out or when you are fighting off a pathogen, the kynurenine pathway will dominate, with less tryptophan available for conversion to serotonin. Kynurenine can bind to the aryl hydrocarbon receptor and regulate immune response.[ref]
The metabolites of kynurenine, or what kynurenine is broken down into, are a key to why the body shunts tryptophan towards this path.
Kynurenine can either be converted into kynurenic acid or quinolinic acid, both of which are important in different ways.
Kynurenic acid from kynurenine:
Kynurenic acid is a glutamate receptor antagonist, which means that it decreases the firing of certain types of neurons. Kynureic acid can also moderate the immune response, keeping the immune response under control.
Interferon-gamma, which is released by the immune system in response to viruses, can cause an increase in kynurenic acid. This increase in kynurenic acid then helps to attenuate inflammatory cytokines (TNF, HMGB1) and keeps the immune response from getting out of control.
Kynurenic acid also has positive and negative effects in the brain. It can block the N-methyl-d-aspartic acid (NMDA) receptor, and can also alter GABA, glutamate, and dopamine levels.[ref] Researchers think that high kynurenic acid in the brain contributes to schizophrenia.[ref]
The flip side of the story is seen with cancer cells. Kynurenic acid secreted by tumor cells may prevent the immune system from recognizing and killing cancer cells.[ref]
Balance is key.
Quinolinic acid from kynurenine:
The second path kynurenin can go down is to become quinolinic acid and then niacin.
Kynurenine can be converted, through a couple of intermediate steps, to quinolinic acid. This is thought to be the route that most kynurenine takes under normal, healthy conditions.[ref]
In general, quinolinic acid is an intermediary. It has negative consequences if it hangs around too long or at higher levels.
What happens with too much quinolinic acid? Quinolinic acid is a neurotoxin that binds to the NMDA receptor. It causes neurodegeneration and apoptosis (cell death).
Quinolinic is unable to pass through the blood-brain barrier, so it is only neurotoxic to the brain when produced in the brain by macrophages or microglial cells. This is true as long as the blood-brain barrier is intact.[ref]
Too much quinolinic acid in the brain is associated with Alzheimer’s disease, ALS, Huntington’s, autism, depression, and suicide attempts. The excess quinolinic acid in the brain causes overactivation of the NMDA receptor. This leads to oxidative stress, not enough energy in the brain, and eventual cell death of the neurons.[ref][ref]
Excess quinolinic acid in the brain reduces mitochondrial energy production. Part of this impact on mitochondrial function may be through an excess of oxidative stress. Additionally, animal research shows that quinolinic acid directly reduces complex II and III in the mitochondria, causing decreased ATP (energy) production.[ref]
Quinolinic acid in the brain can also cause excessive glutamate release, and at the same time, inhibit glutamate reuptake. It also potentiates excitotoxin toxicity.[ref]
Animal studies show that elevating quinolinic acid causes fatigue, similar to the fatigue experienced when ill.[ref]
The link between quinolinic acid and depression has been the subject of quite a few recent research papers.
- A 2016 paper theorizes one cause of depression may be due to tryptophan metabolism being shunted to the kynurenine pathway – which could increase quinolinic acid and, at the same time, decrease serotonin. This shift would be promoted by either an inflammatory response and/or stress hormones, both of which activate the IDO enzymes.[ref][ref]
- There are quite a few studies linking inflammation to causing depression and bipolar disorder. This could be due, at least in part, to kynurenine pathway activation.[ref][ref]
NAD+ production: quinolinic acid to niacin
Quinolinic acid sounds a bit scary, but that isn’t the final step in this pathway. The end point of the kynurenine to quinolinic acid path results in the production of NAD+, which is essential for energy production in cells.
Quinolinic acid is converted by the body into an essential form of niacin called NAD+ (nicotinamide adenine dinucleotide).
In the mitochondria, NAD+ is essential in the production of ATP.
Important here also is that magnesium is a cofactor in the conversion of quinolinic acid to NAD+.
One driving factor for tryptophan becoming quinolinic acid and then NAD+ is a lack of niacin in your diet.
Tryptophan: Conversion to Serotonin and Melatonin
Here’s the pathway image again (focusing on the right side this time):

Your body also uses tryptophan to make the neurotransmitter serotonin. While we often think of serotonin as a happy molecule in the brain, it also acts as a neurotransmitter elsewhere in the body, such as in the intestines, where it regulates gut motility.
- The TPH1 enzyme is the rate-limiting enzyme for producing serotonin in the periphery, such as in the intestines. [ref]
- The TPH2 enzyme is the limiting factor in converting tryptophan into serotonin.
Genetic variants can cause the TPH2 enzyme to function differently, and the variants are linked to a higher relative risk of depression. (Details in the genetics section below)
Crossing the blood-brain barrier:
In the brain, serotonin needs to be made from tryptophan that has crossed the blood-brain barrier. Tryptophan needs a transporter to cross the blood-brain barrier, and that transporter is shared with other branch-chain amino acids. So when tryptophan is consumed along with other proteins, all of it may not reach the brain.[ref]
Consuming carbohydrates, though, can increase the amount of tryptophan that reaches the brain. Eating carbohydrates tends to increase insulin levels, which alters the ratio of tryptophan to other amino acids in the bloodstream. This allows more tryptophan to reach the brain and be converted into serotonin.[ref][ref]
Melatonin:
Serotonin in the brain can be converted to melatonin, a hormone that is important for circadian rhythm, glucose regulation, and sleep. Melatonin is also an important intracellular antioxidant.
Related article: Melatonin: Immune system superstar
Sleep and tryptophan: Increasing tryptophan may increase sleep quality for some people, and this is likely through increasing melatonin levels. A study in elderly adults found that tryptophan added to breakfast cereal increased melatonin levels and improved sleep quality.[ref] Not all studies agree here, and it may be that age plays an important role.[ref] Younger adults naturally produce more melatonin at night, and tryptophan supplementation may not give as big a boost to sleep quality.
Depression: low serotonin or high quinolinic acid?
While it is often thought that decreased serotonin levels in the brain causes depression, the science is not exactly cut-and-dry here. It turns out it is really hard to measure serotonin levels in human brains. There is definitely a connection between biomarkers of serotonin and depression, and increasing serotonin can help with depression for some people… but it isn’t as simple as depression is caused by low serotonin for everyone.[ref][ref]
Researchers have created a mouse model of reduced TPH2 enzyme activity. They have shown that this significantly decreases serotonin levels in the brain and causes mouse depression and anxiety symptoms.[ref] And other researchers have shown that this decreased TPH2 activity causes mice to be susceptible to psychosocial stress.[ref] Decreased serotonin synthesis in adult mice also causes circadian disruption and hyperactivity.[ref] Thus, tryptophan conversion in the brain into serotonin is likely to play some role in mood, anxiety, and circadian rhythm.
Keep in mind that one cause of tryptophan conversion being shunted away from serotonin and towards kynurenine is increased inflammation. This causes a double whammy – lower serotonin and higher quinolinic acid. Quinolinic acid acts on NMDA receptors and too much can kill off brain cells. Studies show that depression scores correlate to higher quinolinic acid in the blood, and postmortem studies show more cells in the brain producing quinolinic acid in suicide victims.[ref]
Don’t forget the gut…
The graphic above shows the various ways the body can convert tryptophan. The cells lining the intestines can convert tryptophan into serotonin using the TPH1 enzyme. Serotonin is thought to be partly responsible for gut motility, and an excess of serotonin increases motility. [ref]
However, the graphic leaves out one other way that tryptophan can be converted — the gut microbiome. Your gut bacteria can also use some of the same enzymes that your cells use in order to convert tryptophan into serotonin or kynurenine metabolites. [ref]
Dietary sources of tryptophan:
The estimated recommended daily intake for adults is between 250- 425 mg/day of tryptophan — which is not a lot. Most people consume more than this amount daily. Common sources of tryptophan in the diet include milk, tuna, chicken, turkey, peanuts, chocolate, oatmeal, bananas, and cheese.[ref]
Is too much tryptophan bad? A study of 29,000 people found that high levels of tryptophan in the diet are not a problem for kidney or liver function. The study did find that higher tryptophan intake correlated to lower levels of depression and better sleep.[ref]
Pellagra: a niacin deficiency disease
I mentioned above that tryptophan is converted to kynurenine, quinolinic acid, and then into NAD+, a form of niacin needed for cellular energy production.
A severe lack of niacin (vitamin B3) causes pellagra, which is a disease that causes the three D’s: diarrhea, dementia, and dermatitis. We get niacin by eating foods that contain it or through converting tryptophan via the kynurenine pathway into niacin.
Symptoms of pellagra include:[ref]
- brain fog (mental confusion)
- weakness
- loss of appetite, abdominal pain
- diarrhea
- inflamed mucous membranes
- scaly skin sores, especially where exposed to the sun
Historically, pellagra was a problem in the southern US after the Civil War due to the nutritional deficiency of niacin from eating a diet mainly consisting of corn. Corn doesn’t contain tryptophan, and the niacin in corn is bound up in such a way that it needs to be nixtamalized (soaked in an alkaline solution) before eating it. This is why native populations in Mexico soak corn with lime water before making the tortillas. This nixtamalization process makes the niacin available and prevents pellagra.
Relying on corn as a dietary staple also had another drawback. Not only does corn lack tryptophan or bioavailable niacin, but it also contains a lot of leucine, a branched-chain amino acid (BCAA). Leucine (and other BCAA) compete with tryptophan for uptake through the blood-brain barrier. Thus, it is thought that high leucine along with low tryptophan contributes to pellagra.
Kynurenine Pathway and Chronic Illnesses:
There are several interesting newer studies on the kynurenine pathway that I want to touch on here. I don’t think the science is completely settled, but current research points to some possibilities that should be considered.
Kynurenine, IDO Metabolic trap, ME/CFS:
There is an interesting hypothesis that tryptophan metabolic trapping is the cause of CFS/ME (chronic fatigue syndrome). You can read the hypothesis, by Drs. Rober Phair and Ron Davis, in full here.
As I understand it, the hypothesis is that CFS/ME is caused by initially activating the kynurenine pathway through interferon and then inhibiting the IDO1 enzyme through too much tryptophan. Excess tryptophan feeds back to inhibit IDO1, and the theory is that this feedback loop then causes high serotonin and low kynurenine. The low kynurenine then leads to low NAD+ and a lack of cellular energy. A genetic component of impaired IDO2 gene function adds to the problem.
An animal study adds an interesting bit of information here. The study shows that a metabolite of serotonin, n-acetyl-serotonin, is a positive allosteric modulator of IDO1. This indicates that with more n-acetyl-serotonin, there is an increase in IDO1 and a decrease in neuroinflammation.[ref]
Kynurnine pathway in COVID-19: Elevated metabolites
Recently, a study published in Nature showed that the kynurenine pathway is intertwined with severe COVID-19. The researchers found that the levels of kynurenine and the kynurenine metabolites were elevated in the urine of people with severe disease. The study looked at all the amino acids in urine and found that the kynurenine metabolites were excessively elevated. The ratio of trytophan to kynurnine revealed that the kynurenine pathway was “drastically increased among patients with COVID-19 as compared with healthy controls.”
The authors of the study explain that interferon-gamma is elevated due to viral infections, which increases the IDO1 enzyme and shunts tryptophan to the kynurenine pathway. This in turn helps to regulate the immune response. However, this also contributes to the viruses ability to evade the immune system initially, allowing for greater viral replication and organ damage. Experimental animal studies showed that inhibiting IDO initially helped to stop the viral infection. [ref]
Another study found similar results with kynurenine, quinolinic acid, and other kynurenine metabolites elevated in SARS-CoV-2 infections. This study also found that trytophan was decreased and nicotinic acid (niacin) was increased. The authors in this study point to quinolinic acid as being a possible cause of fatigue and cognitive issues in Covid patients. [ref]
Kynurenine pathway in Long Covid Brain Fog
A recent preprint explains a study on cognition after mild SARS-CoV-2 infection in an unvaccinated population. The study of 128 people assessed them at 2, 4, and 12 months after Covid. The results showed mild to moderate cognitive impairment, often termed ‘brain fog’, in up to a quarter of the participants. The researchers found that kynurenine pathway metabolites, including quinolinc acid, were elevated in participants with cognitive impairment. The conclusion was that the association suggested “a potential causal link thereby indicating it as a biomarker and therapeutic target”.[ref]
Spike protein, ACE2, and tryptophan metabolism: one more thread
An interesting study used the spike protein from SARS-CoV-2 to investigate changes in tryptophan absorption. The research was done in animals, and it was based on a preliminary study showing that downregulation of ACE2 expression in the intestines causes a reduction in tryptophan absorption. ACE2 is the receptor that the spike protein binds to. In the intestines, ACE2 acts “acts as a regulator of the homeostasis of dietary amino acids, particularly tryptophan”.[ref]
In the cells lining the intestines, ACE2 combines with the Broad Neutral Amino Acid Transporter 1 (B0AT1), which is the transporter used to absorb tryptophan and other neutral amino acids.[ref] In animal studies, ACE2 deletion in the gut increased susceptibility to inflammatory colitis, similar to IBD, and niacin could reverse the inflammation.[ref]
Hidradenitis suppurativa and Kynurenine:
A study of patients with hidradenitis suppurativa, an inflammatory skin condition leading to acne-like nodules or abscesses, showed an interesting link to the kynurenine pathway. The researchers did a metagenomics study on skin biopsy samples from HS patients compared to healthy controls. The sampling showed that they kynurenine pathway and hionolinate were elevated in the skin samples of people with hidradentitis suppurativa.
Obesity and elevated kynurenine:
A recent study found that kynurenine is elevated in obesity. While it has long been known that obesity is associated with increased inflammation, this new study found that the elevation in kynurenine may be more than just a compensatory measure to combat inflammation. Specifically, there is an increase in IDO1 in adipocytes (fat cells). And blocking IDO1 actually stops obesity in an animal model. Additionally, supplementing with vitamin B6, a cofactor for converting kynurnine to its metabolites, also stopped the increase in kynurenine and blocked obesity. [ref]
Tryptophan: Genotype Report
Members: Log in to see your data below.
Not a member? Join here.
Why is this section is now only for members? Here’s why…
Lifehacks for tryptophan conversion:
The rest of this article includes information on supplements for increasing or decreasing the conversion of tryptophan to kynurenine or serotonin. Consider joining today to see the rest of this article.
Related Genes and Topics
Is inflammation causing your depression and anxiety?
Research over the past two decades clearly shows a causal link between increased inflammatory markers and depression. Genetic variants in the inflammatory-related genes can increase the risk of depression and anxiety.
Lithium Orotate + B12: The bee’s knees, for some people…
For some people, low-dose, supplemental lithium orotate is a game-changer when combined with vitamin B12. But other people may have little to no response. The difference may be in your genes.
Serotonin Genes
Serotonin is a neurotransmitter that is important in depression, sleep, and many other aspects of health. Learn how your genetic variants in the serotonin receptor genes impact their function.
Bipolar Disorder, Depression, Circadian Clock Genes
New research shows that depression and bipolar disorder are linked to changes or disruptions in circadian genes. Some people carry genetic variants in the circadian genes that make them more susceptible to circadian disruption.
