Key takeaways:
~ Some types of mold produce mycotoxins, which are toxic at very low levels.
~ Our detoxification pathways eliminate many types of mycotoxins that we are exposed to regularly in low levels.
~ Genetic variants in the detoxification genes make some people more susceptible to negative health effects from mold and mycotoxins.
~ Knowing your genetic susceptibility can help you target the right pathways to detoxify mold and mycotoxins.
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Join today.
Mold and Mycotoxins: Health Effects and Sources of Exposure
We are all exposed to mold on a daily basis. Fungi are essential to life: they live on our skin, make our soil fertile, and help decompose organic matter. But… some molds release microscopic toxins that can harm you. And for some people, exposure to certain types of mold can lead to long-term health problems.
Mold is a general term for the types of multicellular fungi that are filamentous. Yeast is also classified as a fungus, but it is single-celled and doesn’t produce filaments.
Exposure to certain types of mold – the kind that produces mycotoxins – can cause chronic health problems, gut health issues, immediate illness, mitochondrial dysfunction, or even death. When people talk about the health effects of mold, they are usually referring to the effects of mycotoxin exposure.
What are mycotoxins?
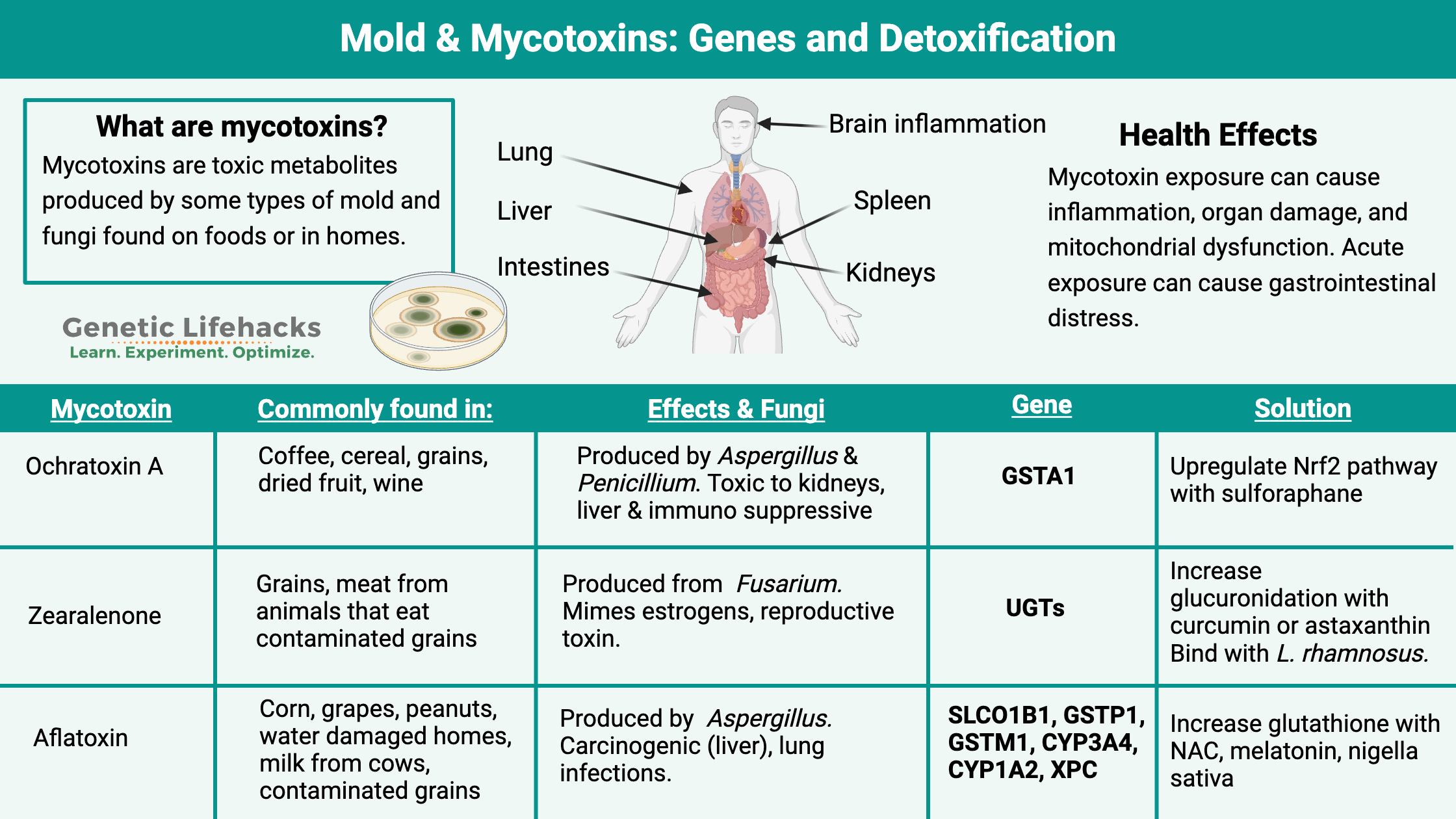
Mycotoxins are microscopic mold metabolites that can cause harm. They are naturally occurring toxins produced by filamentous fungi (molds). They are classified as toxins because even at very low doses, they can cause illness or even death in humans and other animals.[ref] Mycotoxins are different from the toxins found in poisonous mushrooms, which are the fruiting bodies of fungi.
Mycotoxins are found in trace amounts on moldy nuts, grains, coffee, and dried fruits. The mold that grows in damp places, such as water-damaged building materials, can also produce mycotoxins.
Aflatoxins, ochratoxins, trichothecenes, zearalenone, fumonisins, and ergotamine are more commonly studied mycotoxins, but more than 300 mycotoxins are known to exist. Some of these mycotoxins are produced by more than one type of fungus, while others are specific to a single fungal species.[ref]
The World Health Organization estimates that up to 25% of crops are contaminated with mold or fungal growth at some point in their life cycle. Some grains may have fungal problems in the field, while other products may grow mold during storage.[ref] Food processing methods reduce mold or mycotoxin contamination in foods that are used for human consumption. However, animal feed is more commonly contaminated with mold.
How are we exposed to mycotoxins?
Mycotoxins are produced by different types of fungi that grow on crops, in preserved foods, in damp homes, and in the soil, especially where warm and humid conditions are conducive to fungal growth.
The main routes of exposure to mycotoxins include:[ref]
- Ingestion: Contaminated food and drink (nuts, grains, coffee, dried fruit)
- Inhalation: Airborne spores in damp buildings
- Dermal: Skin contact with moldy materials
Foods can be contaminated by mycotoxins either in the field or during drying, storage, and processing. Contamination with mycotoxins is more of a problem when agricultural processes and food handling procedures are poor.[ref]
Mycotoxins pose a dual problem:[ref]
- They are toxic at extremely low levels
- They are chemically stable, allowing them to survive cooking
Mold Exposure: Acute vs. Chronic
Exposure to mycotoxins can be either acute or chronic:
- Acute exposure will result in rapid and severe symptoms of poisoning for someone who is exposed to higher levels of mycotoxins at one time (e.g., eating a bunch of moldy nuts).
- Chronic exposure is more common. Exposure to very low doses over long periods of time can result in various health effects, including an increased risk of certain cancers.
Acute exposure from eating foods containing mycotoxins can cause symptoms of food poisoning. Researchers believe that a percentage of food poisoning cases each year are actually due to mycotoxins.
Chronic exposure to mycotoxins can come from food or a moldy environment. For example, mycotoxins often contaminate coffee beans and tea leaves at very low levels. Drinking contaminated coffee or tea every morning is one way we can be chronically exposed to trace levels of mycotoxins.[ref]
In addition, mycotoxins can be airborne and enter the lungs or pass through the skin. Homes, schools, and office buildings that have been water damaged by flooding, roof leaks, plumbing drips, or AC condensation can develop mold in hidden places, leading to chronic mycotoxin exposure.
~ Mycotoxins are toxic compounds produced by molds, found in food and indoor environments.
~ Exposure can be acute or chronic (low-level, long-term), leading to a range of health effects.
~ Individual susceptibility varies based on genetic differences in detoxification pathways.
Specific mycotoxins: Dive into the details
Mycotoxin is a general term for many different types of toxins, and people are unique in how their bodies process and eliminate the different types of mycotoxins.
Genetic variants in the detoxification genes mean that some people may have little or no problem with mycotoxin exposure, while others may be very sensitive to low amounts of specific mycotoxins. Understanding the different types of mycotoxins, along with understanding your genes, may help you to identify what may be a problem for you.
Let’s take a look at several specific mycotoxins and what they do in the body:
- Ochratoxin A
- Zearalenone
- Aflatoxin A and B1
- Trichothecene
- Fumonisins
- Ergot alkaloids
- Deoxynivalenol
- Patulin
1) Ochratoxin A is produced by Aspergillus and Penicillium sp. Ochratoxin A is toxic and thought to be carcinogenic, especially in the kidneys and liver.[ref]
Ochratoxin A is found in cereals, coffee, wine, dried fruits, beer, and grape juice. It is also found in animal organs (kidneys, liver) of grain-fed animals. In humans, ochratoxin A can have a severe immunosuppressive effect at low and high exposure doses. Ochratoxin A also alters the absorption of nutrients in the intestines.[ref][ref][ref]
2) Zearalenone is produced by Fusarium species that grow on corn or other grains.[ref] It can bind to estrogen receptors (mimics estrogen) and is a reproductive toxin in animal studies. In addition, zearalenone is toxic to the liver and causes cell death.[ref] One study describes zearalenone as: “a non-steroidal compound that exhibits oestrogen-like activity”.[ref]
3) Aflatoxins are produced by a couple of different Aspergillus species. There are 18 types of aflatoxins, with aflatoxin B1 being one of the most toxic and carcinogenic.[ref]
Aflatoxins are commonly found in peanut products and sometimes in milk from cows fed with contaminated grain. Aflatoxin B1 is also found in cottonseed oil.[ref] Aflatoxicosis is the medical term for acute aflatoxin exposure that leads to liver damage, jaundice, and, in severe cases, death.[ref] Chronic dietary exposure to aflatoxins is one cause of liver cancer.[ref]
Aspergillosis is a lung infection caused by Aspergillus infections in the lung. This mainly occurs in immunocompromised people or people with chronic lung conditions.[ref][ref] Aspergillus infection in the lungs or respiratory tract secrete aflatoxins. These aflatoxins then impair the airway cilia from functioning correctly, leading to reduced mucosal clearance.[ref]
4) Trichothecene mycotoxins encompass about 100 subtypes of metabolites from Fusarium species. Trichothecenes can contaminate corn, wheat, barley, oats, rice, rye, vegetables, and other crops. They are a common cause of poisoning in animals eating contaminated feed. Trichothecenes are easily absorbed and then distributed throughout the animal’s tissues. Human exposure comes from consuming meat, milk, and eggs from animals fed contaminated grains.[ref]
Consumption of trichothecene-contaminated foods can cause gastrointestinal issues. This mycotoxin affects actively dividing cells, such as in the intestinal or oral mucosa, and causes cell death.[ref]
5) Fumonisins are metabolites produced by Fusarium fungal species that can grow on peanuts, corn, and grapes. Certain fumonisin subtypes are linked to an increased risk of esophageal cancer, and in general, fumonisins are considered a WHO class 2B carcinogen.[ref][ref]
An interesting observation by researchers is that fumonisins reduce folate uptake in cells: “Because fumonisin B1 reduces uptake of folate in different cell lines, fumonisin consumption has been implicated in neural tube defects in human babies”.[ref]
6) Ergot alkaloids are compounds created by Claviceps species, which are fungal pathogens that attack grasses such as rye. Ergot poisoning has been known for centuries. It was described as a “slow nervous fever” that occurred in the summer after a wet winter in the Middle Ages and was called St. Anthony’s fire. The symptoms recorded throughout history include convulsions, sores, hallucinations or mania, headaches, nausea, gangrene, and burning extremities.[ref] Modern grain processing methods eliminate ergot as a problem in human food sources, but it can still affect animals that graze on grasses or contaminated grains.[ref] The neuroactive components in the ergot alkaloids are similar to precursor molecules for LSD. Interestingly, a couple of Parkinson’s drugs are derived from ergot.
7) Deoxynivalenol is a mycotoxin produced by Fusarium species. It is found in wheat, beans, and some spices. Deoxynivalenol causes severe gastrointestinal issues similar to food poisoning when consumed via contaminated foods.[ref]
8) Patulin is a mycotoxin produced by Aspergillus, Penicillium, and Byssochlamys sp. It is most often found on apples, but can also occur on other moldy fruits or grains. The biggest dietary source is apple juice made from apples that aren’t fresh. Patulin can cause gastrointestinal problems and also organ damage.[ref] Patulin binds to thiol groups found in the intracellular antioxidant glutathione, which then causes oxidative stress, mitochondrial dysfunction, and cell death.[ref]
Health Effects of Mycotoxin Exposure: Symptoms from Mold
Exposure to mycotoxins can produce various physical responses — depending on the toxin, exposure route, amount, and individual genetic differences. Here’s what research shows:
Mitochondrial dysfunction:
This mycotoxin can impair mitochondrial function and inhibit the synthesis of certain proteins.[ref] Even at very low levels, mycotoxins, such as aflatoxin, can cause mitochondrial dysfunction and cell death. In the mitochondria, mycotoxins can cause disruption in the respiratory chain, decreasing the production of ATP and leading to chronic diseases.[ref] Some mycotoxins, such as patulin, can bind to thiol groups in glutathione, thus decreasing this essential cellular antioxidant. Without enough glutathione, oxidative stress and mitochondrial dysfunction.[ref]
Lung inflammation:
An inflammatory response occurs when lung tissue is exposed to airborne trichothecenes. IL-1B increases in a manner dependent on NLRP3 activation.[ref] Aflatoxin G1 airborne exposure significantly increases inflammation in the lung. [ref] Stacchybotry chartarum, also known as black mold, produces trichothecenes that can enter the lungs and cause inflammation and allergic symptoms.[ref]
Organ damage and cell death:
From airborne exposure to fungi, the mycotoxins can travel from the lungs to the liver, kidneys, and spleen. Mycotoxins can cause inflammatory cytokines to increase in all of these organs. Specifically, researchers found higher levels of IL-1B, IL-6, and TNF-alpha.[ref] Genetic variants can influence how strong the inflammatory response may be. For example, TNF-alpha variants may cause some individuals to produce more of this cytokine in response to a mycotoxin. The overactive immune response then causes more cellular damage, exacerbating the problems from the mycotoxins.
Read more about SNPs that cause excessive inflammation
Suppressed immune response:
Zearalenone is a mycotoxin that suppresses the normal inflammatory response that cells should produce against pathogenic bacteria. Zearalenone exposure tamps down the proinflammatory cytokines (TNF-alpha, IL1B, IL6) that should be produced in response to gram-negative bacteria. This ‘tamping down’ suggests a reduction in the innate immune response, which could leave someone vulnerable to infections after exposure to zearalenone.[ref]
Kidney damage:
Oral exposure to ochratoxin A from contaminated food is linked to kidney injury. Animal studies show that ochratoxin A increases inflammatory cytokines and upregulates genes related to fibrosis in the kidneys. Blocking NLRP3, an inflammatory activator, was able to suppress the kidney injury.[ref] Patulin is also known for causing kidney damage. The mechanism of action here seems to be mitochondrial damage and lack of ATP — kidney cells use a lot of ATP. [ref]
Gut microbiome interaction:
Exposure to mycotoxins through foods can impact the gut microbiome in several ways. First, some gut microbes can metabolize mycotoxins, sometimes creating more toxic metabolites. Mycotoxins can also impact the composition of the gut microbiome by killing off some bacteria and affecting gut barrier function. Interestingly, certain mycotoxins affect the way nutrients are absorbed.[ref][ref]
Atopic dermatitis (eczema):
Long-term exposure to visible mold during infancy increases the risk of atopic dermatitis in children.[ref]
Asthma:
Many epidemiological studies link childhood asthma to mold exposure.[ref] One study looked at the interaction between genetic variants linked to asthma and mold exposure. In children exposed to visible mold, such as on the ceiling, there was a three-fold increased risk of asthma when taking into account genetic variants and mold exposure.[ref]
Detoxifying mycotoxins: pathways involved
We are all regularly exposed to mycotoxins at trace levels, and our bodies have ways of getting rid of mycotoxins. The key is to not overwhelm the detoxification system through either an excess of toxins or by not having enough of the cofactors needed for detoxification.
In general, the body breaks down and eliminates toxins in three steps: phase I detoxification (CYP450 genes) makes the substance more polar; phase II detoxification (UGTs, GSTs) makes the metabolite water-soluble; and phase III is the excretion of the metabolite.
| Detox Phase | Main Genes/Enzymes | Function |
|---|---|---|
| Phase I | CYP450 (CYP1A2, CYP3A4) | Makes toxins more polar/reactive |
| Phase II | UGT, GST, SULT | Conjugates toxins for excretion |
| Phase III | Transporters (ABCs) | Excretes water-soluble metabolites |
Let’s look at some of the detoxification pathways involved in mycotoxin elimination:
Aflatoxin detoxification:
Aflatoxin can be combined in the body with glutathione, making it easy for the body to excrete. Having enough glutathione available to handle mycotoxins is essential, and the GST family of genes is important here.[ref]
Ochratoxin A:
Exposure to ochratoxin A increases oxidative stress. It can be counteracted on a cellular level by the Nrf2 pathway, which is necessary for upregulating genes that combat reactive oxygen species.[ref]
Zearalenone:
Glucuronidation is a phase II detoxification process by which a glucuronic acid molecule is added to a toxin to make it more easily excreted and less reactive. Zearalenone mycotoxins are excreted via the glucuronidation pathway, specifically utilizing UGT1A1, UGT1A3, and UGT1A8.[ref]
Additionally, methylation, hydroxylation (addition of a hydroxyl group), hydrolysis, and sulfation reactions are utilized for transforming mycotoxins for excretion from the body.[ref]
Table: Major Mycotoxins, Sources, Health Effects, and Detoxification Pathways
| Mycotoxin | Main Sources | Health Effects | Pathways/Genes |
|---|---|---|---|
| Ochratoxin A | Cereals, coffee, wine, dried fruits, animal organs | Carcinogenic (kidneys/liver), immunosuppression, nutrient absorption issues | Nrf2 pathway, GST, UGT |
| Zearalenone | Corn, grains | Estrogenic, reproductive toxicity, liver toxicity | UGT1A1, UGT1A3, UGT1A8 |
| Aflatoxins | Peanuts, milk, cottonseed oil | Liver damage, cancer, immunosuppression | GST, CYP1A2, CYP3A4, XPC |
| Trichothecenes | Corn, wheat, barley, oats, animal products | GI issues, cell death, immunosuppression | CYP450, GST |
| Fumonisins | Corn, peanuts, grapes | Esophageal cancer, neural tube defects, hepatotoxic | Folate pathways, CYP450 |
| Ergot Alkaloids | Rye, grains | Neurological symptoms, gangrene, hallucinations | CYP450 |
| Deoxynivalenol | Wheat, beans, spices | GI issues, cell death | GST, UGT |
| Patulin | Apples, apple juice, fruits | GI problems, organ damage, oxidative stress | Glutathione, GST |
Mold Genes: Are the HLA types in 23andMe?
I want to take a moment to do a bit of ‘myth busting’ here. One question that I get asked fairly often is how to find the mold genes in 23andMe data. There is a lot of confusion and oversimplification being promoted on the internet about “mold genes” (sometimes from practitioners selling tests and supplements).
Some natural health practitioners write about mold genes and refer to certain HLA types (HLA-DRB1, HLA-DRB3, HLA-DRB4, and HLA-DRB5). These HLA types are pretty common — found in over a quarter of the population. About 20 years ago, practitioners found that people with these HLA types were over-represented in having mold reactions. They described symptoms of mold illness include fatigue, muscle pain, headaches, sinus problems, vision problems, brain fog, mood swings, vertigo, joint pain, weakness, and more.
The only published research on the HLA genes and mold symptoms is a couple of case studies.[ref][ref] While these HLA types may have a link to being more prone to an immune system reaction from mold exposure, I am not finding anything other than a link between asthma, HLA types, and mold sensitivity in children.[ref]
Does a lack of peer-reviewed research mean that people don’t really have mold illness? Of course not. Clinicians make valid discoveries about health all the time, and not everything is written in a peer-reviewed journal. However, I’m sticking with just explaining what high-quality research studies show.
What does research show as far as the connection between genes and mycotoxin detoxification?
There are quite a few studies on the genetic variants involved in reactions to individual mycotoxins. Most of these variants are related to the detoxification pathways.
Phase I detoxification involves the modification of toxins by cytochrome P450 enzymes in the liver, making them more polar and reactive. Phase II detoxification follows, where these modified toxins are conjugated with molecules like glucuronide, sulfate, or glutathione, making them water-soluble and easier to excrete from the body
CYP1A2 and CYP3A4 are important phase I detoxification genes for a couple of types of aflatoxins. The UGT and GST family of genes are important for the phase II detoxification of several different types of mycotoxins. Variants that decrease gene function here are pretty common.
Aflatoxin B is a known carcinogen (can cause cancer). A DNA repair gene, XPC, interacts with aflatoxin B1 in the risk of liver cancer.
Mycotoxin Reaction Genotype Report:
Lifehacks for Mold: Supplements, environmental factors
The big picture with mycotoxins is avoidance when possible, and then counteracting the negative effects of exposure.
Let’s look at multiple ways of doing this that are backed by research studies.
Avoiding mold contamination:
First up, let’s look at what research shows about environmental factors for mold exposure from what you eat, where you live/work, and medications you regularly take.
Dietary sources of mold
In general, food production and storage are important in avoiding mold contamination, especially in humid climates. Here are some of the well-studied dietary sources to consider:
- Coffee: Older studies show that mycotoxin contamination was fairly common in coffee.[ref][ref] However, newer testing shows that many organic brands of coffee are both mycotoxin and pesticide-free. Tested brands included Peet’s, DeathWish, Bulletproof, and 12 more.[article] A 2022 study found that 28% of green coffee beans (not roasted) had ochratoxin A contamination, but only 4% of the roasted coffees contained mycotoxin.[ref]
- Peanuts and peanut butter: Peanuts grow where it is warm and humid. They are prone to fungal growth and are a big source of aflatoxin exposure.[ref][ref]
- Pork products: Pigs that are fed grains contaminated with ochratoxin A are a dietary source of ochratoxin for people who regularly eat pork products.[ref] Many countries do periodic testing for contamination, so this isn’t a problem with all pork in all areas. However, this is a reason to choose high-quality pork or pork from a known local farmer when possible.
- Your coffee maker: Research shows that the coffee pot reservoir and basket or pods with wet grounds are also sources of daily bacterial and fungal exposure. Aspergillus species thrive on caffeine.[ref][[ref][ref]
- Bee pollen supplements: Pollen, such as bee pollen supplements, has been shown to harbor aflatoxins, ochratoxins, fumonisins, zearalenone, and other mycotoxins.[ref]
- Grains: There’s more of a risk for mycotoxin contamination in grains that have been improperly stored. Most commercially available grains and flours are free of mycotoxins, but not all. For example, ConsumerLab.com found that Bob’s Red Mill Organic Whole Grain Buckwheat contained low levels of zearalenone.
- Liver supplements: Often taken for liver health, 28% of milk thistle supplements were shown in one study to have a high concentration of mycotoxins.[ref]
Want to test a food or supplement yourself for mycotoxins? SimpleLab has an easy way to order tests (but it’s pricey!)
Check your home and office for mold:
Water damage is notorious for causing mold growth, and moldy buildings can be a continual source of airborne mycotoxin exposure. Look for leaking plumbing, water from an air conditioner condenser, or small roof leaks.
Here are a few studies on airborne mycotoxins in buildings:
- A study in a school in Malaysia found that children had more headaches, runny noses, and tiredness when in classrooms with higher levels of mycotoxins in the dust.[ref]
- School HVAC systems in the US are also a significant source of mold exposure.[ref]
- HEPA filters in air purifiers are actually a source of the molds that can produce mycotoxins. A study found Aspergillus, Penicillium, Alternaria, Cladosporium, Trichoderma, and other fungal species in the filters after 6 to 12 months in homes.[ref]
- Wallpaper that gets wet (such as in the bathroom) can easily grow mold, and moving air, such as from the bathroom fan, can easily aerosolize the mycotoxins.[ref]
- Other wet building materials that support mold growth include Sheetrock, chipboard, and spruce wood.[ref]
- Washing machines, especially around the lid/door and the detergent tray, can be a big source of fungal and bacterial biofilms.[ref] Sanitize your washing machine regularly, and while you’re cleaning, also run your dishwasher on a sanitize cycle.
Mold test kits are available online, and there are often local companies that specialize in mold testing. Testing may help you pinpoint which mycotoxin is causing your mold symptoms.
Cleaning up your environment:
While mycotoxins are resistant to extreme temperatures, UV light can eliminate them.[ref][ref] With mild-intensity UV light, zearalenone and deoxynivalenol are eliminated in an hour. More UV intensity breaks them down faster.
Ozone also breaks down or alters many mycotoxins to decrease toxicity.[ref][ref]
Drugs that may slow down detoxification:
Mycotoxins may be impossible to avoid completely, but most people have no problem with low-level exposure. However, if you are impairing your detoxification pathways with prescription medications, it could be adding to your mold problems.
- UGT inhibitors include the drug class of kinase inhibitors[ref]
- Clindamycin (antibiotic) inhibits GST enzyme activity[ref]
~ Store food in dry, cool places to prevent mold growth.
~ Choose tested brands for coffee and peanuts.
~ Regularly clean coffee makers and washing machines.
~ Inspect homes for water damage and mold.
~ Consider genetic testing for detoxification gene variants if you have persistent symptoms.
Supplement to improve mycotoxin detoxification pathways:
Let’s look at how to target oxidative stress from mycotoxins in general, and then zoom in on the specific supplements and diet changes for genetic variants.
Article Recap: Mold, Mycotoxins, and Your Genes
- Mycotoxins are widespread environmental toxins with diverse health effects.
- Genetic differences in detoxification pathways explain why some people are more sensitive.
- Understanding your genetic profile can help tailor prevention and detox strategies.
- Practical steps—food choices, home hygiene, and awareness—can reduce risk for everyone.
Related Articles and Topics:
Rheumatoid Arthritis: Genetics, Root Causes, and Treatment Research
References:
Ahmed Adam, Mowaffaq Adam, et al. “Effects of Different Mycotoxins on Humans, Cell Genome and Their Involvement in Cancer (Review).” Oncology Reports, vol. 37, no. 3, Mar. 2017, pp. 1321–36. PubMed, https://doi.org/10.3892/or.2017.5424.
Al-Ahmad, Mohammad M., et al. “Genetic Polymorphisms of Cytochrome P450-1A2 (CYP1A2) among Emiratis.” PLoS ONE, vol. 12, no. 9, Sept. 2017, p. e0183424. PubMed Central, https://doi.org/10.1371/journal.pone.0183424.
Amuzie, Chidozie J., et al. “Tissue Distribution and Proinflammatory Cytokine Induction by the Trichothecene Deoxynivalenol in the Mouse: Comparison of Nasal vs. Oral Exposure.” Toxicology, vol. 248, no. 1, June 2008, pp. 39–44. PubMed, https://doi.org/10.1016/j.tox.2008.03.005.
Awuchi, Chinaza Godseill, et al. “Mycotoxins’ Toxicological Mechanisms Involving Humans, Livestock and Their Associated Health Concerns: A Review.” Toxins, vol. 14, no. 3, Feb. 2022, p. 167. PubMed Central, https://doi.org/10.3390/toxins14030167.
Bennett, J. W., and M. Klich. “Mycotoxins.” Clinical Microbiology Reviews, vol. 16, no. 3, July 2003, pp. 497–516. DOI.org (Crossref), https://doi.org/10.1128/CMR.16.3.497-516.2003.
Cai, Peirong, et al. “Molecular Mechanism of Aflatoxin-Induced Hepatocellular Carcinoma Derived from a Bioinformatics Analysis.” Toxins, vol. 12, no. 3, Mar. 2020, p. E203. PubMed, https://doi.org/10.3390/toxins12030203.
Deng, Jiang, et al. “Aflatoxin B1 Metabolism: Regulation by Phase I and II Metabolizing Enzymes and Chemoprotective Agents.” Mutation Research. Reviews in Mutation Research, vol. 778, Dec. 2018, pp. 79–89. PubMed, https://doi.org/10.1016/j.mrrev.2018.10.002.
el Khoury, André, and Ali Atoui. “Ochratoxin A: General Overview and Actual Molecular Status.” Toxins, vol. 2, no. 4, Mar. 2010, pp. 461–93. PubMed Central, https://doi.org/10.3390/toxins2040461.
Guilford, Frederick T., and Janette Hope. “Deficient Glutathione in the Pathophysiology of Mycotoxin-Related Illness.” Toxins, vol. 6, no. 2, Feb. 2014, pp. 608–23. PubMed Central, https://doi.org/10.3390/toxins6020608.
Ito, Miyabi, et al. “Functional Characterization of 20 Allelic Variants of CYP1A2.” Drug Metabolism and Pharmacokinetics, vol. 30, no. 3, June 2015, pp. 247–52. PubMed, https://doi.org/10.1016/j.dmpk.2015.03.001.
Janik, Edyta, et al. “T-2 Toxin—The Most Toxic Trichothecene Mycotoxin: Metabolism, Toxicity, and Decontamination Strategies.” Molecules, vol. 26, no. 22, Nov. 2021, p. 6868. PubMed Central, https://doi.org/10.3390/molecules26226868.
Jaramillo-Rangel, G., et al. “Polymorphisms in GSTM1, GSTT1, GSTP1, and GSTM3 Genes and Breast Cancer Risk in Northeastern Mexico.” Genetics and Molecular Research: GMR, vol. 14, no. 2, June 2015, pp. 6465–71. PubMed, https://doi.org/10.4238/2015.June.11.22.
Klein, Kathrin, and Ulrich M. Zanger. “Pharmacogenomics of Cytochrome P450 3A4: Recent Progress Toward the ‘Missing Heritability’ Problem.” Frontiers in Genetics, vol. 4, Feb. 2013, p. 12. PubMed Central, https://doi.org/10.3389/fgene.2013.00012.
Knutsen, A. P., et al. “Mold-Sensitivity in Children with Moderate-Severe Asthma Is Associated with HLA-DR and HLA-DQ.” Allergy, vol. 65, no. 11, Nov. 2010, pp. 1367–75. PubMed, https://doi.org/10.1111/j.1398-9995.2010.02382.x.
Lee, Po-Yen, et al. “Mycotoxin Zearalenone Attenuates Innate Immune Responses and Suppresses NLRP3 Inflammasome Activation in LPS-Activated Macrophages.” Toxins, vol. 13, no. 9, Aug. 2021, p. 593. PubMed, https://doi.org/10.3390/toxins13090593.
Lee, Su-Jun, and Joyce A. Goldstein. “Functionally Defective or Altered CYP3A4 and CYP3A5 Single Nucleotide Polymorphisms and Their Detection with Genotyping Tests.” Pharmacogenomics, vol. 6, no. 4, June 2005, pp. 357–71. PubMed, https://doi.org/10.1517/14622416.6.4.357.
Li, Hu, et al. “Ochratoxin A Induces Nephrotoxicity in Vitro and in Vivo via Pyroptosis.” Archives of Toxicology, vol. 95, no. 4, Apr. 2021, pp. 1489–502. PubMed, https://doi.org/10.1007/s00204-021-02993-6.
Li, Peng, et al. “Detoxification of Mycotoxins through Biotransformation.” Toxins, vol. 12, no. 2, Feb. 2020, p. 121. PubMed Central, https://doi.org/10.3390/toxins12020121.
Liew, Winnie-Pui-Pui, and Sabran Mohd-Redzwan. “Mycotoxin: Its Impact on Gut Health and Microbiota.” Frontiers in Cellular and Infection Microbiology, vol. 8, Feb. 2018, p. 60. PubMed Central, https://doi.org/10.3389/fcimb.2018.00060.
Long, Xi Dai, et al. “XPD Codon 312 and 751 Polymorphisms, and AFB1 Exposure, and Hepatocellular Carcinoma Risk.” BMC Cancer, vol. 9, Nov. 2009, p. 400. PubMed, https://doi.org/10.1186/1471-2407-9-400.
Long, Xi-Dai, Yun Ma, et al. “Polymorphism in Xeroderma Pigmentosum Complementation Group C Codon 939 and Aflatoxin B1-Related Hepatocellular Carcinoma in the Guangxi Population.” Hepatology (Baltimore, Md.), vol. 52, no. 4, Oct. 2010, pp. 1301–09. PubMed, https://doi.org/10.1002/hep.23807.
Long, Xi-dai, et al. “[Study on the detoxication gene gstM1-gstT1-null and susceptibility to aflatoxin B1 related hepatocellular carcinoma in Guangxi].” Zhonghua Liu Xing Bing Xue Za Zhi = Zhonghua Liuxingbingxue Zazhi, vol. 26, no. 10, Oct. 2005, pp. 777–81.
Long, Xi-Dai, Hong-Dong Huang, et al. “XPC Codon 939 Polymorphism Is Associated with Susceptibility to DNA Damage Induced by Aflatoxin B1 Exposure.” International Journal of Clinical and Experimental Medicine, vol. 8, no. 1, 2015, pp. 1197–204.
Malir, Frantisek, et al. “Transfer of Ochratoxin A into Tea and Coffee Beverages.” Toxins, vol. 6, no. 12, Dec. 2014, pp. 3438–53. PubMed Central, https://doi.org/10.3390/toxins6123438.
Mellon, J. E., et al. “Influence of Lipids with and without Other Cottonseed Reserve Materials on Aflatoxin B(1) Production by Aspergillus Flavus.” Journal of Agricultural and Food Chemistry, vol. 48, no. 8, Aug. 2000, pp. 3611–15. PubMed, https://doi.org/10.1021/jf0000878.
Nock, Nora L., et al. “Polymorphisms in Glutathione S-Transferase Genes Increase Risk of Prostate Cancer Biochemical Recurrence Differentially by Ethnicity and Disease Severity.” Cancer Causes & Control, vol. 20, no. 10, 2009, pp. 1915–26. PubMed Central, https://doi.org/10.1007/s10552-009-9385-0.
Peng, Qiliu, et al. “Association between XPD Lys751Gln and Asp312Asn Polymorphisms and Hepatocellular Carcinoma Risk: A Systematic Review and Meta-Analysis.” Medicine, vol. 93, no. 29, Dec. 2014, p. e330. PubMed, https://doi.org/10.1097/MD.0000000000000330.
Pfeiffer, Erika, et al. “Glucuronidation of Zearalenone, Zeranol and Four Metabolites in Vitro: Formation of Glucuronides by Various Microsomes and Human UDP-Glucuronosyltransferase Isoforms.” Molecular Nutrition & Food Research, vol. 54, no. 10, Oct. 2010, pp. 1468–76. PubMed, https://doi.org/10.1002/mnfr.200900524.
Piacentini, Sara, et al. “GSTA1*-69C/T and GSTO2*N142D as Asthma- and Allergy-Related Risk Factors in Italian Adult Patients.” Clinical and Experimental Pharmacology & Physiology, vol. 41, no. 3, Mar. 2014, pp. 180–84. PubMed, https://doi.org/10.1111/1440-1681.12201.
Reljic, Zorica, et al. “Is Increased Susceptibility to Balkan Endemic Nephropathy in Carriers of Common GSTA1 (*A/*B) Polymorphism Linked with the Catalytic Role of GSTA1 in Ochratoxin a Biotransformation? Serbian Case Control Study and in Silico Analysis.” Toxins, vol. 6, no. 8, Aug. 2014, pp. 2348–62. PubMed, https://doi.org/10.3390/toxins6082348.
Richards-Waugh, Lauren L., et al. “Fatal Methadone Toxicity: Potential Role of CYP3A4 Genetic Polymorphism.” Journal of Analytical Toxicology, vol. 38, no. 8, Oct. 2014, pp. 541–47. PubMed, https://doi.org/10.1093/jat/bku091.
Shao, Peilu, et al. “Aflatoxin G1 Induced TNF-α-Dependent Lung Inflammation to Enhance DNA Damage in Alveolar Epithelial Cells.” Journal of Cellular Physiology, vol. 234, no. 6, June 2019, pp. 9194–206. PubMed, https://doi.org/10.1002/jcp.27596.
“St. Anthony’s Fire.” World History Encyclopedia, https://www.worldhistory.org/St_Anthony’s_Fire/. Accessed 20 Apr. 2022.
Tijhuis, Mariken J., et al. “GSTP1 and GSTA1 Polymorphisms Interact with Cruciferous Vegetable Intake in Colorectal Adenoma Risk.” Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, vol. 14, no. 12, Dec. 2005, pp. 2943–51. PubMed, https://doi.org/10.1158/1055-9965.EPI-05-0591.
Wong, John, et al. “Lung Inflammation Caused by Inhaled Toxicants: A Review.” International Journal of Chronic Obstructive Pulmonary Disease, vol. 11, June 2016, pp. 1391–401. PubMed Central, https://doi.org/10.2147/COPD.S106009.
Xia, Qing, et al. “Review on Contaminants in Edible Oil and Analytical Technologies.” Oil Crop Science, vol. 6, no. 1, Mar. 2021, pp. 23–27. ScienceDirect, https://doi.org/10.1016/j.ocsci.2021.02.001.
Yang, Haiyan, et al. “The Association of GSTM1 Deletion Polymorphism with Lung Cancer Risk in Chinese Population: Evidence from an Updated Meta-Analysis.” Scientific Reports, vol. 5, Mar. 2015, p. 9392. PubMed, https://doi.org/10.1038/srep09392.
Zain, Mohamed E. “Impact of Mycotoxins on Humans and Animals.” Journal of Saudi Chemical Society, vol. 15, no. 2, Apr. 2011, pp. 129–44. ScienceDirect, https://doi.org/10.1016/j.jscs.2010.06.006.