Key takeaways:
~ Osteoarthritis causes joint pain, stiffness, and joint instability in the knees, hands, hips, and spine.
~ Elevated inflammatory cytokines, due to injuries or aging, cause cartilage degradation in the joints.
~ Genetic variants in inflammation- and collagen-related genes increase the risk of osteoarthritis.
~ Understanding which variants you have may help you to target the right pathways.
Osteoarthritis: Underlying Causes
Osteoarthritis is a condition that causes chronic joint pain. Affected joints often include the knees, hands, hips, and spine.
The joints most commonly affected are the knees, hands, hips, and spine.
More than one-third of adults over the age of 60 show evidence of osteoarthritis on X-rays, but not all will experience significant joint pain.[ref]
Risk factors for osteoarthritis include certain genetic variants, age, weight, and joint misalignment.
Symptoms of osteoarthritis include joint pain, stiffness, joint instability, and narrowing of the joint space on x-ray.
Current treatment options usually include exercise, weight loss, and pain medications. For more severe cases of osteoarthritis, hip or knee replacement surgery is an option.
I’m going to dive into what is causing the joint problems, looking at the pathophysiology driving osteoarthritis.
Goal:
By understanding the processes and genes involved, my hope is that you can figure out which natural solutions (Lifehacks section) will work best with your genes to alleviate the pain and hopefully reverse the arthritis processes.
Background science: What happens in the joints in arthritis?
Cartilage is the flexible, firm connective tissue that protects your joints in three ways, according to the Cleveland Clinic:[ref]
- Absorbs shock, like a cushion between your bones
- Reduces friction, allowing bones to glide without rubbing
- support structure, keeping your joints in shape
Joint cartilage is composed primarily of type II collagen, tissue fluid, and proteoglycans. Other types of collagen and proteins make up a small percentage of the cartilage composition.
The collagen and proteoglycans form a dense mesh of fibrils that are then hydrated by the tissue fluid. [ref]
Cells called chondrocytes secrete and make up the joint cartilage network.
Injury or Aging: Progression of osteoarthritis
Cartilage in the joints can be damaged by injury or normal wear and tear. At the onset of osteoarthritis, the surface of the cartilage is undamaged, but the organization of the extracellular matrix is altered.
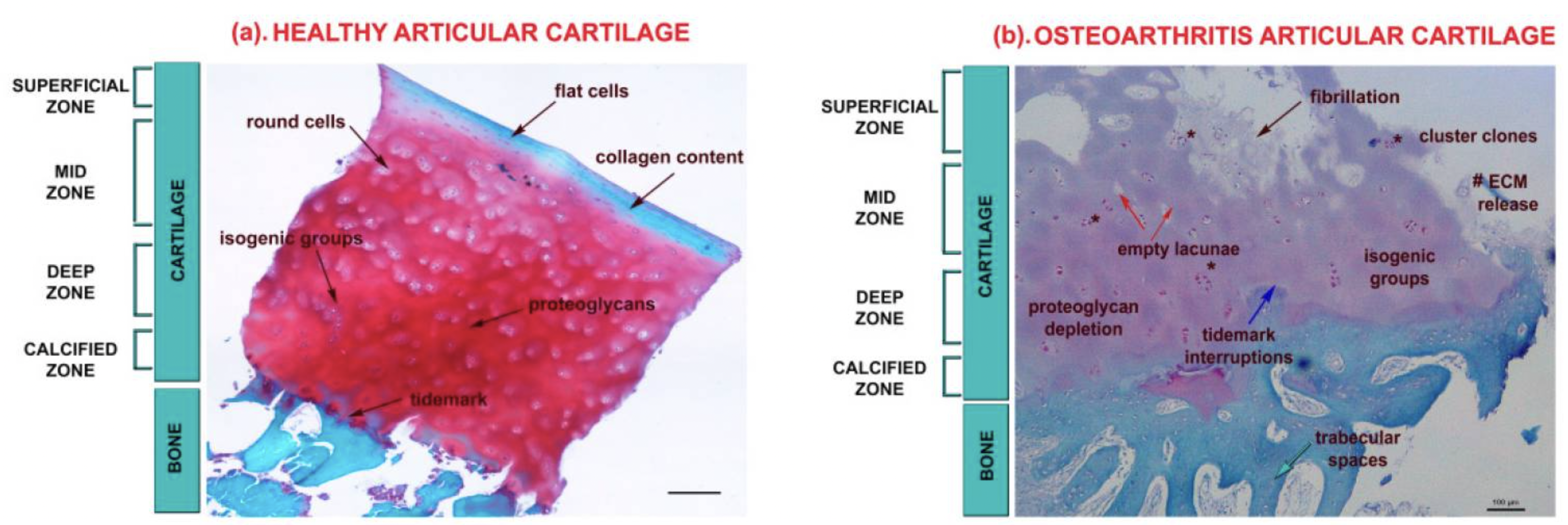
Chondrocytes, the cells that make up cartilage, don’t regenerate very easily. Instead, when damage occurs, these cells can undergo apoptosis (cell death), and the cartilage can be lost. Without cartilage, there is friction between the bones, leading to pain and joint instability.[ref]
That’s usually where the explanation of arthritis stops — cartilage breaks down and the joint hurts… But recent research is giving us a much better understanding of what goes on inside the joint.
Osteoarthritis vs. inflammatory arthritis:
Osteoarthritis is usually distinguished from inflammatory arthritis by the amount of morning stiffness. People with osteoarthritis usually have less than 30 minutes of morning stiffness and less systemic inflammation.[ref] Inflammatory arthritis includes rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis.
Traditional treatments for inflammatory arthritis, such as corticosteroids, hydroxychloroquine, and methotrexate, are usually not effective for osteoarthritis. Additionally, the biologic therapies used for rheumatoid arthritis aren’t usually effective for osteoarthritis.[ref]
Inflammatory pathways in osteoarthritis:
The lack of efficacy of anti-inflammatory drug trials put osteoarthritis in the “not inflammatory” camp — until recently.
Research now shows that innate immune activation may be important in osteoarthritis. Genetic studies show that variants in genes related to IL-1 (interleukin 1) and TNF-alpha can increase the risk of osteoarthritis. Additionally, genes related to the formation and structure of collagen are also involved in the risk of osteoarthritis. These two pathways interact, with inflammation driving changes in the collagen.
Upregulated inflammatory pathways:
In osteoarthritis, IL-1β, TNF-α and IL-6 are upregulated. This leads to upregulation of signaling pathways that stimulate other inflammatory factors that promote disease progression.[ref]
Mechanical wear and tear injuries can cause inflammation that drives IL-1B, TGF-Beta, and NF-kB. Animal studies show that inhibiting the activation of the NF-kB pathway protects against osteoarthritis. [ref][ref]
NF-kB signaling and miRNA (microRNA) alterations cause the chondrocytes, or the joint cartilage cells, to begin producing enzymes that break down the extracellular matrix. Specifically, matrix metalloproteinase-13 (MMP-13) has been identified as being activated to degrade type II collagen in joints.
TNF-alpha, inflammatory cytokine:
TNF-alpha is also upregulated in osteoarthritis. TNF-alpha also activates the NF-kB pathway, which increases other inflammatory factors and degradation of the extracellular matrix. Additionally, the synthesis of type II collagen in chondrocytes is blocked by TNF-alpha.[ref]
IgE-mediated mast cell activation:
Studies show that mast cells are activated in the synovial fluid in the joints of people with osteoarthritis. Mast cells are part of the innate immune response and can be activated by allergens, pathogens, or inflammatory cytokines.
Mast cell activation has also been found in animal studies of osteoarthritis. Importantly, mice that lack mast cells are significantly protected against the induction of osteoarthritis.[ref]
Animal studies also show that the trigger for mast cell activation in osteoarthritis is IgE-mediated. Researchers explain that breakdown products from cartilage can activate mast cells via the IgE pathway.[ref] However, a study of over 100,000 people found that people with allergies and asthma (IgE-mediated diseases) are at an increased risk of osteoarthritis, showing a possible link to allergens as well.[ref]
Collagen breakdown:
Matrix metalloproteinases are enzymes that break down collagen in the extracellular matrix. In general, low levels of MMPs promote healthy tissue remodeling, but in osteoarthritis, there may be excess production of MMPs that break down collagen.[ref]. MMP inhibitors have been developed, but they have too many off-target side effects to be useful.[ref]
MMP1 and MMP13 are specific metalloproteinases that play a role in the breakdown of cartilage collagen in osteoarthritis. This ties back into the increase in inflammation as well. Inflammatory cytokines increase MMP1 and MMP13 expression.[ref]
Gut microbiome:
Researchers have linked dysbiosis of the gut microbiome to osteoarthritis. This may be because gut dysbiosis causes low-grade inflammation or changes in the immune system. [ref] Other researchers have found bacterial nucleic acid in the joint synovial fluid of people with osteoarthritis.[ref]
Excessive iron absorption:
Iron levels are tightly regulated in the body because excess iron can cause oxidative stress. One place where the body can store excess iron is in the joints. A recent Mendelian randomization study found that genes associated with higher transferrin saturation levels are causative for osteoarthritis.[ref][ref]
Can osteoarthritis be reversed?
For centuries, it was thought that cartilage didn’t heal once it was destroyed in osteoarthritis.
However, improved MRI imaging shows that about 30% of cartilage defects spontaneously repair themselves over time. Additionally, a 14-year radiographic study of knee osteoarthritis showed that it sometimes regresses.[ref] The key here is that regression or reversal of cartilage damage in osteoarthritis is a slow process.
There is a lot of research and trials going on to find better solutions for osteoarthritis. Researchers are looking at cell and gene therapies, tissue regeneration, and growth factors. For example, treating knee cartilage by injecting chondrocytes is often used in younger osteoarthritis patients. [ref]
Initially, inflammation wasn’t thought to play a role in osteoarthritis, in part because therapies that just block IL-1B didn’t work all that well. Newer research and genetic studies have shown that inflammation and IL-1B are involved. Instead of just targeting IL-1B, a multipronged approach that decreases IL-1B, TNF-alpha, and the inflammatory cascade may be needed.[ref]
Osteoarthritis Genotype report
Inflammation-related genes:
IL1RA gene: encodes the receptor for interleukin 1, which is pro-inflammatory
Check your genetic data for rs3917225 (23andMe v4; AncestryDNA):
- A/A: typical
- A/G: less likely to have osteoarthritis
- G/G: less likely to have osteoarthritis[ref]
Members: Your genotype for rs3917225 is —.
IL1B gene: encodes interleukin-1B, an inflammatory cytokine. Excessive IL-1B response also increases the inflammatory damage seen in pancreatitis.
Check your genetic data for rs1143634 C3954T (23andMe v4, v5; AncestryDNA):
- A/A: increased inflammationref][ref][ref]; increased risk of osteoarthritis[ref]
- A/G: increased risk of osteoarthritis
- G/G: typical
Members: Your genotype for rs1143634 is —.
TNF gene: encodes TNF-alpha, an inflammatory cytokine. Variants that increase TNF production increase the risk of pancreatitis. In-depth TNF article.
Check your genetic data for rs1800629 -308A/G (23 and Me v4, v5; AncestryDNA):
- A/A: Higher TNF-alpha levels. Increased risk of osteoarthritis[ref]
- A/G: somewhat higher TNF-alpha levels
- G/G: typical
Members: Your genotype for rs1800629 is —.
IL-17F gene: inflammatory cytokine, mainly produced by T helper 17 immune system cells
Check your genetic data for rs763780 G7488A (23andMe V5 only):
- T/T: typical
- C/T: increased risk of osteoarthritis
- C/C: increased risk of rheumatoid arthritis; Hasimoto’s.[ref][ref][ref] increased risk of osteoarthritis[ref][ref]
Members: Your genotype for rs763780 is —.
Hemochromatosis (iron-overload) genes:
Excess iron is linked to an increased risk of osteoarthritis.[ref]
HFE Gene: The most common type of hemochromatosis is Type 1, or Classic, usually caused by variants in the HFE gene.
Check your genetic data for rs1800562 C282Y (23andMe v4, v5; AncestryDNA):
- A/A: two copies of the C282Y variant, most common cause of hereditary hemochromatosis, highest ferritin levels; increased risk of osteoarthritis[ref][ref]
- A/G: one copy of C282Y, increased ferritin levels, hemochromatosis possible but less likely[ref], increased risk of osteoarthritis
- G/G: typical
Members: Your genotype for rs1800562 is —.
Bone and joint-related variants:
VDR gene: encodes a vitamin D receptor
Check your genetic data for rs1544410 (23andMe v4, v5; AncestryDNA):
- C/C: more common genotype; osteoarthritis more frequent
- C/T: decreased relative risk of osteoarthritis
- T/T: carrier of BsmI variant, possible increased risk of low bone mineral density[ref][ref], decreased relative risk of osteoarthritis[ref][ref]
Members: Your genotype for rs1544410 is —.
Check your genetic data for rs731236 (23andMe v4, v5; AncestryDNA):
- G/G: typical[ref]
- A/G: increased risk of osteoarthritis[ref]
- A/A: increased risk of osteoarthritis[ref]
Members: Your genotype for rs731236 is —.
CKM gene: encodes creatine kinase
Check your genetic data for rs4884 (23andMe v4, v5; AncestryDNA):
- G/G: less likely to have osteoarthritis[ref]
- A/G: less likely to have osteoarthritis
- A/A: typical
Members: Your genotype for rs4884 is —.
HIF1A gene: hypoxia-inducible factor 1a, important in vascular function
Check your genetic data for rs11549465 Pro582Ser (23andMe v4, v5; AncestryDNA):
- C/C: typical
- C/T: decreased risk of knee osteoarthritis
- T/T: decreased risk of knee osteoarthritis[ref]
Members: Your genotype for rs11549465 is —.
Collagen Breakdown:
ADAMTS14 gene: encodes ADAMTS proteinase which is important in degrading the extracellular matrix. Specifically, ADAMTS14 is involved in the collage II fiber formation process[ref]
Check your genetic data for rs4747096 (23andMe v4; AncestryDNA):
Members: Your genotype for rs4747096 is —.
ADAM12 gene: encodes a metalloproteinase that is upregulated by TNF-alpha
Check your genetic data for rs1871054 (23andMe v4, AncestryDNA):
- C/C: increased risk of osteoarthritis[ref]
- C/T: typical risk
- T/T: typical risk
Members: Your genotype for rs1871054 is —.
MMP1 gene: encodes a collagenase that breaks down type 1 collagen
Check your genetic data for rs1799750 (23andMe v4, v5)
- I/I or CC: increased risk of osteoarthritis, higher rate of collagen breakdown [ref]
- D/I: increased risk of osteoarthritis, higher rate of collagen breakdown
- D/D: typical risk
Members: Your genotype for rs1799750 is —.
Lifehacks: Natural Solutions and Personalized Approach for Osteoarthritis
As always, talk with your doctor about any supplement or medical questions. If you are taking prescription drugs, you may also want to talk to your pharmacist about interactions with dietary supplements.
This section covers:
- Physical therapy options (everyone)
- Targeting inflammatory cytokines (inflammation-related genetic variants)
- Repairing cartilage (bone and collagen-related variants)
- Reducing excess iron (hemochromatosis variant)
Physical therapy, exercise therapy, or acupuncture:
Talk with your doctor about physiotherapy options. Both manual physical therapy and exercise therapy have been shown to be effective in improving pain and outcomes in osteoarthritis.[ref]
A clinical trial also showed that acupuncture can be effective in improving pain and function in osteoarthritis.[ref]
Natural ways to combat inflammation in osteoarthritis: