Key takeaways:
~ Obesity rates were steady for decades, and then started rising sharply around 1980.
~ There are multiple causes of obesity and infection with adenovirus 36 is clearly shown in research to be one cause.
~ Research studies clearly show that adenovirus 36 infection of adipose tissue causes slow weight gain for a portion of the population.
~ Genetics plays a role in susceptibility to viruses as well as variation in the genes related to the creation of fat cells.
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Consider joining today.
What causes obesity? Is there a viral component?
When looking at the research on obesity, one thing becomes clear: it’s complicated!
For years we have been told to eat less and exercise more. While that is healthy-sounding advice, it doesn’t seem to be curing the obesity epidemic. According to the World Health Organization, obesity has doubled since 1980. Worldwide in 2014, 1.9 billion adults were overweight.[ref]
It’s almost like obesity has spread through the world like a viral epidemic…
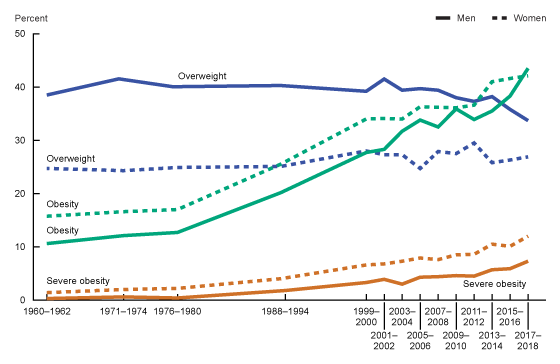
The green line below shows the percentage of people who are obese taking a sharp trend upward around 1980.
About 70% of people in the US are now classified as overweight or obese. This article digs into one cause of obesity – a specific adenovirus.
Can obesity be triggered by a virus?
It seems odd to think about infectious diseases causing obesity. But an abundance of evidence shows that, for some people, this is a cause. Specific adenovirus strains have links to increased fat mass and obesity in humans and other animals.
You may be thinking, like I was — “Really? A virus causing obesity? I don’t think so! People are just lazy and overeating.”
However, there is an abundance of high-quality, replicated research studies that show that a specific species of adenvirus infects fat cells and causes long term changes in adiposity and metabolism.
Let’s dig into the science of this…
Background on adenoviruses:
Adenovirus is a family of viruses with many different species causing a variety of symptoms in humans and other animals.
Human adenoviruses are non-enveloped, double-stranded DNA viruses. They replicate in the nucleus of the host’s cells by hijacking the host’s cellular replication proteins.
Virologists have identified more than 50 adenovirus types causing human infections, and most are spread by direct contact. There are also a bunch of other adenoviruses that only cause illness in other animals, like dogs or monkeys. Examples of illnesses caused by adenoviruses include respiratory illnesses (common cold) in humans and kennel cough in dogs (Canine adenovirus 2).
In humans, different types of adenoviruses can also cause gastroenteritis, croup, viral pink eye, bronchitis, pneumonia, and more.[ref] Thus, adenoviruses are both varied and very common.
Gene therapy and vaccines:
You may be thinking that adenovirus sounds familiar. The Johnson & Johnson and AstraZeneca Covid vaccines use an adenovirus that is modified to deliver the DNA of the SARS-CoV-2 spike protein. The specific virus used in the J&J vaccine is adenovirus 26, which doesn’t cause symptoms of illness in humans. AstraZeneca’s Covid vaccine uses a chimpanzee adenovirus to deliver the spike protein.
Adenoviruses are also used extensively in research for gene therapy to deliver edited genes.[ref] None of these are the species of adenovirus that is known to cause increased fat mass.
Adenoviruses that trigger fat accumulation:
Two species of the adenovirus (Ad-5 and Ad-36) have been linked for decades to adipogenesis, or the creation of fat cells, in humans and animals. This causes weight gain in people and is linked to an increased risk of obesity.[ref]
While it seems counterintuitive that a virus can cause obesity, let me explain a few of the hundreds of studies showing not only a link but also causation of increased fat accumulation due to adenovirus-36 (Ad-36) or Ad-5. (Most studies are on Ad-36, but there are a handful that also looked at Ad-5.)
Animal studies show us the mechanism:
The first animal studies on adenovirus and obesity were done in the 1990s. The avian adenovirus was determined to cause significant fat mass gain in chickens while at the same time decreasing cholesterol and triglyceride levels.[ref] Subsequent studies identified Ad-36 as the likely cause of weight gain in humans.[ref] This kicked off decades of animal studies to determine how and why Ad-36 increases adiposity.
Giving the human adenovirus-36 to monkeys causes a >3-fold body weight gain.[ref]
Interestingly, animal studies show that the gain in fat mass is not due to eating more or moving less… Animals ate the same amount of chow, but those infected with adenovirus 36 increased fat tissue by 60-80%.[ref]
Another study revealed that Ad36 increased inflammatory cytokines (TNFα and MCP1) in adipose cells, but yet improved glycemic control.[ref]
From a series of well-designed animal studies, researchers have discovered that Ad-36 upregulates the expression of several genes, including C/EBPα and PPARγ, which leads to an increase in adipogenesis, resulting in more fat cells.[ref] Essentially, the body can handle more glucose if it can easily store it in fat cells, and the ability to create more fat keeps glucose levels under control.
Digging into the details further, animal experiments show that the E4orf1 protein that is encoded by a gene in Ad-36 holds the key. Ad-36 enters the nucleus of fat cells and replicates its genes using the host cell’s replication mechanisms. One Ad-36 protein that gets replicated is E4orf1, which then acts in the fat cells to increase glucose uptake. Importantly, the increased glucose uptake via GLUT4 receptors is independent of insulin.[ref]
Let’s take a look at the human studies on this to see how it plays out in real life.
Human studies on adenoviruses and obesity:
An initial study in twins:
A 2004 twin study showed that the Ad-36 antibody-positive twins were “significantly heavier and fatter as compared with their antibody-negative counterparts”. This was a groundbreaking study indicating that the Ad-36 virus was likely causing obesity.[ref]
Studies in children:
Researchers found that children with obesity were 4 times more likely to have adenovirus-5 (Ad-5) antibodies. The results of the study showed that 28% of obese children carried the Ad-5 antibodies vs. 6% of non-obese children. The same study also found a significant link between Ad-36 antibodies in obese children (27%) vs. non-obese children (10%).[ref]
A Turkish study replicates those results for adenovirus-36, with 27% of obese children carrying antibodies and 6% of non-obese children. In adults, the researcher found 18% of obese adults had Ad-36 antibodies, compared with 4% of non-obese adults. This is a 4-fold increase in obesity for the Ad-36 group.[ref]
Meta-analysis of multiple studies in adults and children:
When combining the data from 24 different studies on adenovirus 36 and obesity in populations around the world, the results show a very strong association between viral infection and obesity. Overall, Ad-36 increases the risk of obesity by 77% in adults and 126% in children.[ref] More recent studies add to this data.[ref][ref][ref]
PCR testing showing higher viral loads in obese adipose tissue:
A 2023 study took adenovirus research one step further and did PCR testing on adipose tissue. The study found that 83% of the obese people had adenovirus 36 in their fat cells (5-fold increased risk). Moreover, the viral load was 25% higher in people with obesity compared to normal-weight people who were also infected with the virus.[ref]
Not all studies agree: population group differences
While almost all studies show a positive link between Ad-36 and obesity, there are exceptions. A recent study in a Chinese Han population group did not find that Ad-36 was associated with obesity.[ref] Additionally, vitamin D levels seem to play a role in the relationship between Ad-36 and weight gain.[ref][ref]
Adenovirus infections have doubled since the mid-90s:
A study in Sweden replicated the previous results (~2-fold increase in Ad-36 in obese vs. lean). This study, though, also looked back at historical adenovirus data. The presence of Ad-36 positivity doubled from the mid-90s to the mid-2000s, which parallels the increase in obesity during that period.[ref]
Chicken virus, also?
An adenovirus that infects chickens, SMAM-1, was the first one that researchers identified in the 1990s as causing fat gain in chickens. Those same researchers also found that about 20% of the obese people that they screened (in India) had antibodies showing a prior infection with SMAM-1.[ref] I’m not finding subsequent studies on SMAM-1 in humans, so this may be something only found in people in India who had exposure to infected chickens.
Tradeoffs: Pros and cons of Ad-36 infection
Adenovirus 36 infects adipose tissue and alters metabolism there. It upregulates the production of new fat cells, which leads to a greater ability to store glucose as fat.
Research studies on fat gain from adenoviruses show that infection by the virus increases cellular glucose uptake. In addition to adenovirus infection causing increased adipocytes, it also increases GLUT4, a glucose receptor.[ref]
The mechanism used by the virus to create more host cells (more fat) is one that researchers are looking at for drug development for diabetes medications.[ref]
Both animal and human studies show that this has both benefits and drawbacks.
Plus side: decreased risk of diabetes
Adenovirus 36 infection may improve glycemic control, which decreases the risk of diabetes. The virus upregulates the PI3K and GLUT4 pathways, causing pre-adipocytes to take up more glucose. This increases fat mass while simultaneously decreasing blood glucose levels.
Another possible positive here is that animal studies show that the Ad36 infection may help to slow the onset of Alzheimer’s disease. One risk factor in Alzheimer’s is altered glucose in the brain, and the Ad36 infection helps with overall glycemic control. [ref]
Not all is good, though, in regard to the effects of Ad-36. In addition to promoting fat accumulation, it also increases a couple of inflammatory markers, including TNF-alpha and MCP-1.[ref]
Obesity epidemic timing:
If the adenovirus infections are playing a role in our obesity epidemic, this would need to be due to infections rising, starting around the early 1980s, when obesity levels started steadily increasing.
I mentioned above that obesity has doubled since 1980 — and that adenovirus seropositivity had doubled by the mid-2000s (Swedish study).
Here’s the CDC graph again – looking at the green lines (dashed green is women and solid green is men)

One piece of the puzzle as to why this could be happening is the increase in kids in daycare, which has been rising since the 80s. A study looking at overweight kids (9-12) found that Ad-36 increased the risk of obesity by 3-fold and that kids who were in daycare before age 24 months were also at an almost 3-fold risk of obesity.[ref]
Another interesting puzzle piece is that Ad36 was first isolated in 1978 in Germany.[ref] (This may not mean that it first existed in 1978, though, just that it wasn’t well-known before then. Other adenoviruses were identified in the 1930s.)
However, it is likely not as simple as just an increased spread of the adenovirus among kids starting in the 80s. Instead, it is likely that many factors, such as easy access to food, combine together with an increase in the ‘obesity virus’.
Context: Putting the impact of Ad-36 into perspective
Adenovirus-36 infection doesn’t seem to cause obesity in everyone, although the study using PCR tests to show higher AD36 viral loads in adipose tissue of obese people infected with the virus is compelling and suggests that individual viral response may be a component.[ref]
Let’s look at some statistics to put the risk of obesity from adenovirus infection into perspective. Keep in mind some studies show a 200% to 500% increase in the relative risk of obesity with Ad-36 infection, more conservative estimates are around 80% increased relative risk in adults and 130% increased relative risk in children.
The obvious reason for obesity is eating too much sugar and refined carbohydrates. But statistically how much does this add to the risk of obesity?
A 2019 meta-analysis of different food intake studies and weight gain showed that consumption of refined grains increased the relative risk of obesity by 5% and the consumption of sugar-sweetened beverages increased the relative risk of obesity by 10%. Eating more legumes, fruit, and fish all tilted the scales in the other direction, reducing the risk of obesity by 5-15%. The study concluded, “The dose-response meta-analytical findings provided very low to low quality of evidence that certain food groups have an impact on different measurements of adiposity risk.”[ref]
The other reason most people assume is causing the rise in obesity is a lack of physical activity. A Cochrane review of studies on physical activity and BMI in teenagers showed that there was very little evidence showing that increasing physical activity made much of a difference in BMI.[ref] There are lots of other good reasons to stay physically active, though.
What about the increase in consumption of omega-6 seed oils since 1980? A 10-year-long prospective study of women who were initially of normal body weight found that the risk of becoming overweight was increased with a higher omega-6 to omega-3 fat ratio. For women with the highest omega-6:omega-3 ratio, the increased relative risk of being overweight was ~50%. [ref]
My takeaway:
Adenovirus infection statistically increases the risk of obesity quite a bit more than eating sugar, refined carbs, not exercising, or eating too much omega-6 fat. However, stacking all of those reasons together likely adds up to quite a bit of weight gain as well. There is no simple answer, but rather is a complex picture of multiple reasons for weight gain.
With more than three decades of solid research studies on adenovirus infection increasing weight in humans (and animals) – with a relative risk showing it to be quite a bit more important than sugar or fat consumption – why isn’t there a drive to find solutions? Why isn’t this being talked about by doctors and health coaches?
Genotype Report for Adenovirus Susceptibility:
While your genes play a significant role in which viruses are likely to infect you, there isn’t a lot of direct research on adenoviruses, obesity, and genetics. Instead, I’ll focus here on genetic variants that impact Ad-36 susceptibility. Do keep in mind the recent study showing that adipose tissue from obese people with Ad-36 had higher viral loads, so there is likely more than simple susceptibility to the virus involved here.[ref]
Lifehacks:
You may be wondering why you don’t hear a lot about a viral cause of obesity. It’s a fair question, and after looking at the research, I’m wondering why no one seems to be looking for a cure that works for this specific cause of obesity.
One doctor theorized that: “The government and insurance companies do not want to admit that obesity is a disease because then they would have to cover treatment as they do for other diseases.”[ref] This seems to be shifting and obesity is being talked about as a disease, but only in reference to the new GLP-1 medications for weight loss.
Related Articles and Topics: