Key takeaways:
~ Testosterone is an essential hormone for both men and women, and imbalances can lead to health problems.
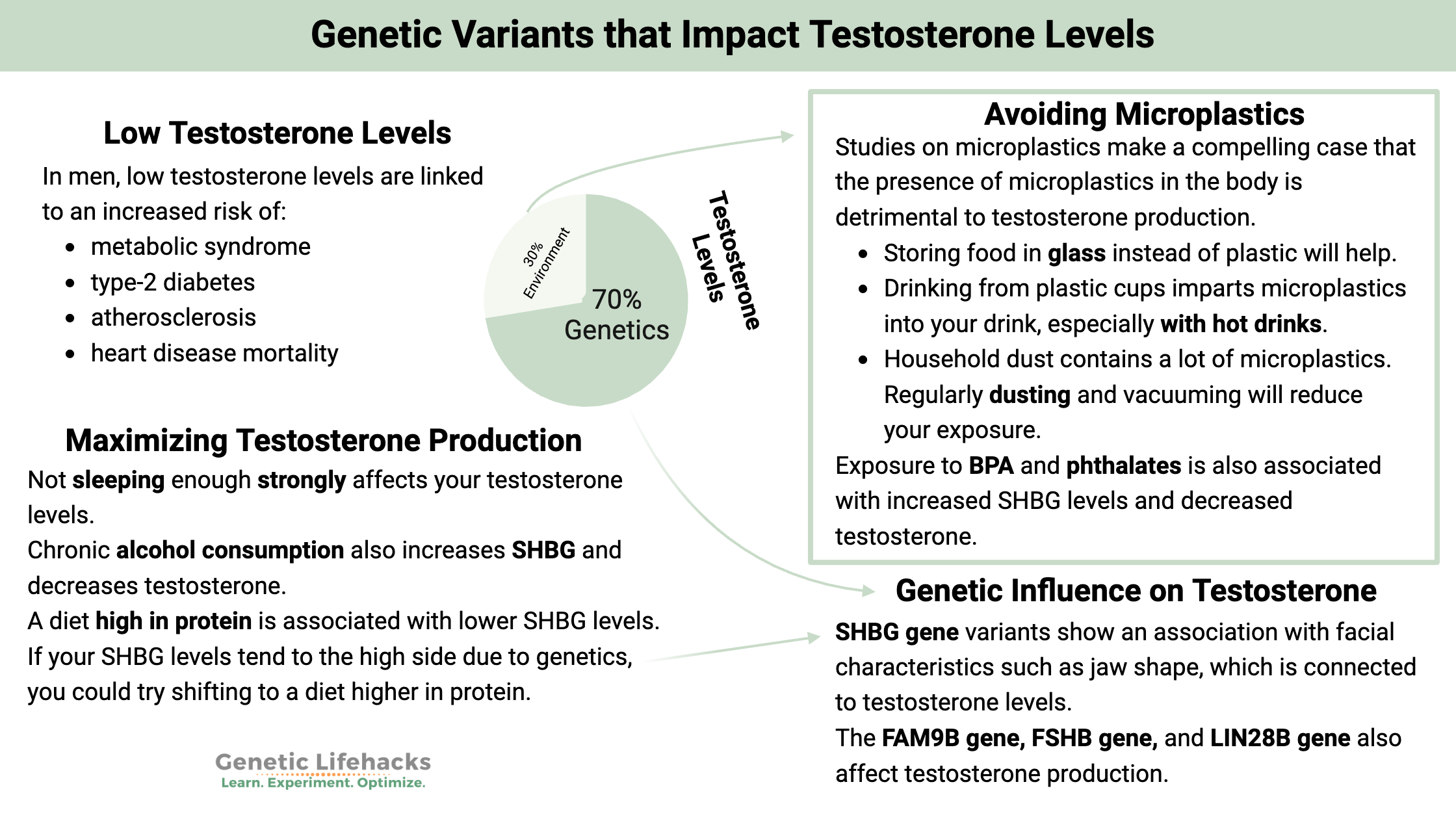
~ Genetics play a significant role in testosterone levels, with an estimated heritability of up to 70% in men.
~ Addressing lifestyle factors such as getting enough sleep, reducing microplastic exposure, eating right, and managing inflammation can help optimize testosterone levels.
This article explains how testosterone is synthesized in the body, how your genetic variants influence free T levels, and how you can make simple changes to increase your testosterone levels if they are low.
Members will see their genotype report below and the solutions in the Lifehacks section. Consider joining today.Testosterone Levels:
Testosterone is an important hormone for men and women of all ages. Like most hormones, testosterone needs to be balanced – not too low and not too high.
Men’s testosterone levels:
In men, low serum testosterone levels are associated with an increased risk of:[ref][ref]
- Metabolic syndrome
- Type-2 diabetes
- Atherosclerosis
- Heart disease mortality
In men, the testes release testosterone when stimulated by luteinizing hormone. Leydig cells in the testes convert cholesterol to produce most of the testosterone in men.
Women’s testosterone levels:
In women, increased testosterone levels are a risk factor for:[ref][ref][ref]
- Fatty liver disease
- PCOS, insulin resistance, and higher fasting glucose levels
- Type 2 diabetes
In women, the adrenal glands and the ovaries produce testosterone in small amounts.
Bound vs. free testosterone:
Most of the testosterone circulating in the bloodstream, about 98%, is bound to either albumin or sex hormone-binding globulin (SHBG). The 2% that is “free” testosterone is the biologically active form that can act on cellular receptors.
Your circulating testosterone levels depend on many factors, including genetics.
What does testosterone do in the body?
Testosterone is a hormone that affects sexual development, muscle strength, bones, health, cognitive function, and more. It works by binding to the androgen receptor (AR), which acts as a transcription factor.
Testosterone binds to the androgen receptors in the cytoplasm of certain types of cells. Once bound to the receptor, the hormone can translocate into the nucleus of the cell and regulate gene transcription for specific androgen-sensitive genes.[ref]
In addition to turning on growth-related genes in the cell nucleus, testosterone also has non-genomic effects as a signaling molecule.[ref]
Testosterone can also be converted to dihydrotestosterone (DHT) by an enzyme called 5α-reductase. DHT can also activate the androgen receptor, and it has a higher binding affinity for the receptor (it’s stronger).[ref]
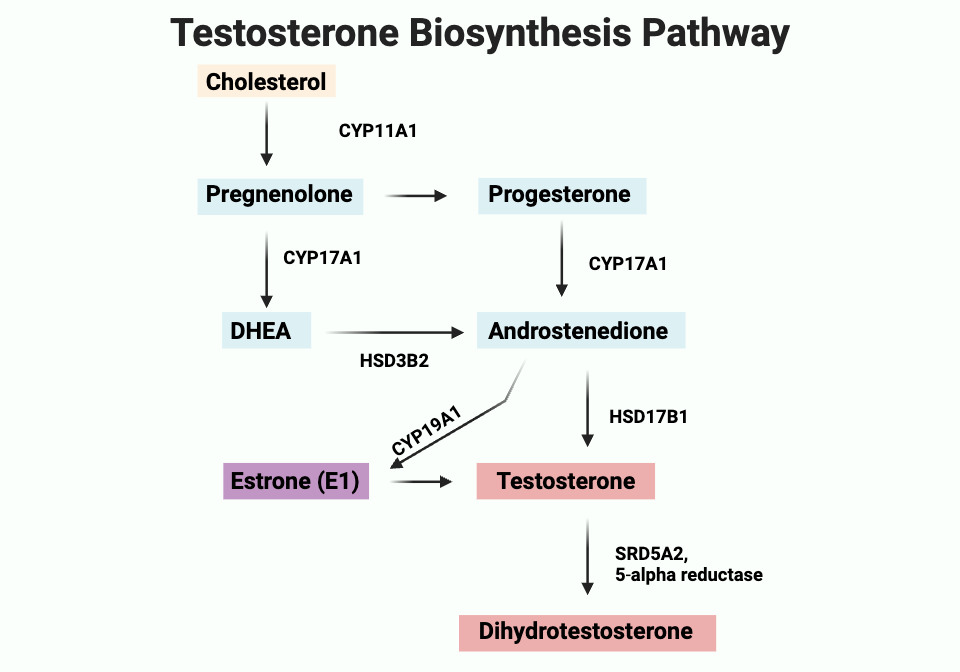
How is testosterone synthesized?
Testosterone is synthesized from cholesterol via a pathway involving progesterone or DHEA, which is converted to androstenedione and then testosterone. Testosterone can then be converted to DHT by the enzyme 5-alpha reductase.
Testosterone homeostasis: How T levels are regulated
I mentioned above that most testosterone is bound to either sex hormone binding globulin (SHBG) or albumin. SHBG effectively controls the amount of testosterone available for active use in the body. Higher levels of SHBG mean less free testosterone is available, and lower levels of SHBG usually mean more active testosterone is available.
SHBG levels tend to rise with age in men, which is one reason why free testosterone levels decrease a bit in older men. Studies show that HFN-4α may be the key to this increase. HFN-4α (HNF4A gene) is a transcription factor that turns on and off genes related to lipid metabolism in the liver as well as other liver functions.[ref][ref]
How do your genes influence testosterone levels?
While age, diet, and lifestyle choices play a role in testosterone levels, there is also a fairly strong genetic factor at play. Studies of male siblings estimate that the genetic component of testosterone levels is ~70%.[ref][ref]
Genetic studies can now use the known variants associated with testosterone levels to see if these variants are associated with other conditions.
For example, studies of the SHBG gene variants show an association with facial features such as jaw shape, which is linked to testosterone levels.[ref]
Other studies link genetic variants that affect testosterone levels to an increased risk of prostate cancer, increased bone mineral density, and lower body fat.[ref]
Sex hormone binding globulin:
Sex hormone binding globulin (SHBG) is a steroid hormone (e.g. testosterone) transport protein produced in the liver. Approximately 44% of testosterone is bound to SHBG, with most of the rest bound to albumin and a small amount (~2%) circulating freely.
Free testosterone and albumin-bound testosterone are more readily available for use in the body, but SHBG-bound testosterone is sequestered. In this way, SHBG controls the amount of testosterone available for use in the body.[ref]
Genetic variants in the SHBG gene- see the Genotype Report section below- can affect the amount of testosterone available in the body.
Androgen receptor variants:
In addition to the measurable testosterone and DHT levels, variants in the androgen receptor also play a role in whether lower levels of testosterone cause someone to have low T symptoms.
There is a repeated segment in the DNA that codes for the AR gene. This repeat segment varies in length from 7 to 36 repeats. In women, the AR repeats may play a role in PCOS (polycystic ovary syndrome), which is caused by higher androgen levels.[ref]
In men, the combination of lower testosterone levels (but still within the normal range) along with a low AR repeat length is more likely to give rise to symptoms of low testosterone.[ref]
What is a normal testosterone level?
There’s quite a bit of variation in what is considered normal for testosterone levels.
In men:
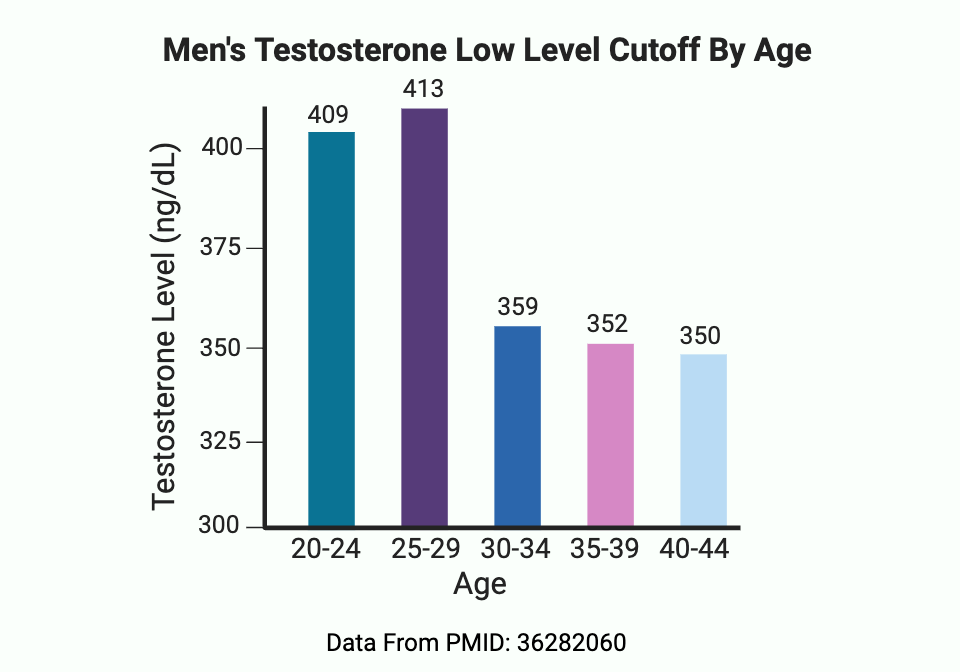
A 2022 study found that: “Age-specific middle tertile levels were 409-558 ng/dL (20-24 years old), 413-575 ng/dL (25-29 years old), 359-498 ng/dL (30-34 years old), 352-478 ng/dL (35-39 years old), and 350-473 ng/dL (40-44 years old). Age-specific cutoffs for low testosterone levels were 409, 413, 359, 352, and 350 ng/dL, respectively.” [ref] However, some studies show that testosterone levels do not decrease much after age 30 and are fairly steady into old age.[ref]
Why is there such a huge range?
The normal range is really wide when looking at tests for men’s testosterone levels. One reason for this is because the time of day (circadian rhythm) causes testosterone levels to vary a lot, and because eating – especially eating carbohydrates or sugar – can cause up to a 25% decrease in levels.[ref] Another reason is that there is a seasonal change in average testosterone levels.[ref] Therefore, the normal range takes into account that testosterone levels go up and down a lot. More on this in the Lifehacks section.
In pre-menopausal women ages 18-49, average testosterone levels are between 15-46 ng/dL.[ref]
The average total testosterone level in post-menopausal women is around 32 ng/dL (1.12 nmol/L). [ref]
Breast cancer risk increases by 18% for every 0.5 nmol/L (14 ng/dL) increase over average testosterone levels in post-menopausal women but not in premenopausal women.[ref]
Symptoms of low testosterone in men:
Some of the clinical features of low testosterone in men include:[ref]
- Fatigue
- Low mood
- Erectile dysfunction
- Reduced muscle mass
- Decreased or low libido
- Loss of early morning erections
- New-onset or increasing gynecomastia
- Osteoporosis
In the Lifehacks section below, I explain the options for testing, how to increase testosterone naturally, and the clinical trials on testosterone replacement therapy
Declining levels for decades:
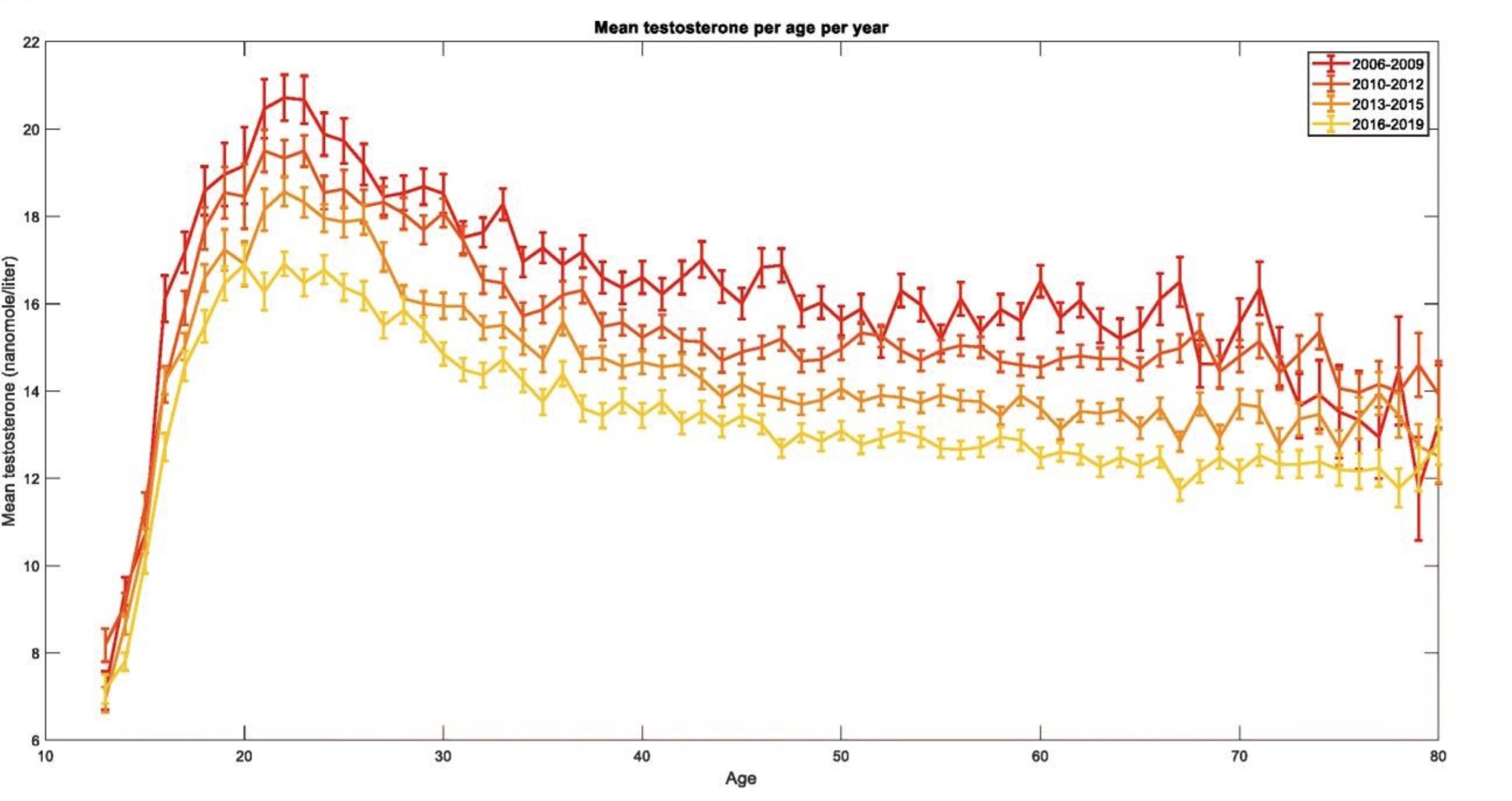
Testosterone levels have been declining for men over the past 50+ years. This decline is seen worldwide and is seen both in normal-weight and overweight men. The trend is in both young and old men, and it is not related to the increase in obesity. It is also likely not due to a shift from saturated fat to PUFA.[ref][ref][ref][ref]
Below is a screenshot from one recent study on the topic. The top red line is the average levels in 2008, with the lower yellow line representing average levels in 2018.
Microplastics and testosterone:
Exposure to plastic is ubiquitous in our modern environment, but the full extent of the problems posed by the microscopic bits of plastic we consume is only now being uncovered.
Microplastics are a problem for testosterone in at least three ways:
- Adsorption of free testosterone
- Endocrine disruption
- Testicular damage
1) Adsorption of testosterone by microplastic:
Apparently, it has been known for more than 5 decades in research labs that steroid hormones are adsorbed (adhered to) by plastic containers.[ref] If you think about storing food in plastic containers, they often will get stained by specific foods (e.g. tomatoes) or can absorb odors (e.g. onion). A similar thing happens with steroid hormones being adsorbed to plastics.
Recently, researchers looked at whether the tiny bits of plastic that ingest each day also adsorb hormones circulating in the body. First, the researchers centrifuged different types of microplastics with a solution of testosterone to see which plastics adsorbed the most hormone. They then used animals to see the in vivo effect of two different types of microplastics in two different sizes. The results showed that polyamide microplastics (e.g. from fabrics) were particularly good at adsorbing circulating testosterone like little sponges – enough to make a measurable difference in testosterone levels within hours due to the plastic particles adsorbing free testosterone. They also found that the plastic particles were taken up by the testicles within 24 hours.[ref]
2) Endocrine disruptor:
Microplastics and nanoplastics also can leach endocrine-disrupting chemicals, such as BPA and phthalates, into the body. A recent study showed that microplastics can reduce the expression of the enzymes needed for testosterone production, such as 17β-hydroxysteroid dehydrogenase (HS17B1 gene in the synthesis diagram above). Polystyrene nanoplastics have also been shown to disrupt luteinizing hormone levels (and LH is a driver of testosterone levels). Another study showed that under inflammatory conditions, the polystyrene microplastics had an even stronger negative effect on testosterone production.[ref][ref][ref]
3) Damage to the testes:
Microplastic and nanoplastic particles are absorbed in the intestines and circulate throughout the body before being taken into various organs. One place where the microscopic particles end up is in the testes. Studies show that they can accumulate in the different cell types there, including damaging the Leydig cells that produce the majority of male testosterone.[ref][ref][ref][ref]
Uncommon genetic conditions that cause low testosterone:
Klinefelter syndrome is caused by having two X chromosomes and one Y chromosome (XXY). It is the most common sex chromosome disorder, affecting about 1 in 600 men.
In general, men with Klinefelter’s have lower testosterone levels resulting in less body hair, less muscle mass, and possibly an abnormal development of the testicles. Only about 25-40% of men with Klinefelter syndrome are ever diagnosed with it, and it is usually discovered during fertility evaluations.[ref] 23andMe does not report on Klinefelter syndrome or other chromosomal abnormalities, and even whole genome sequencing, such as sequencing.com, doesn’t always provide the information.[ref][ref]
Cystic fibrosis is a genetic condition that causes changes to a chloride channel important in the lungs, intestines, and testes. Low testosterone is found in about a third of men with cystic fibrosis.[ref][ref] You can check to see if you are a carrier of a cystic fibrosis mutation here.
Other rare genetic causes of low testosterone include Kallmann Syndrome, congenital growth hormone deficiency, Noonan syndrome, and congenital adrenal hyperplasia. These rare genetic conditions are often identified at birth or during childhood.[ref][ref][ref]
Genotype Report: Testosterone
Note: Other gene variants influence testosterone levels that aren’t available via 23andMe or AncestryDNA testing. Specifically, a commonly repeated section of the androgen receptor plays a significant role in testosterone levels.
Lifehacks: Optimizing testosterone
What is the best way to know if your testosterone levels are normal? Get a blood test to see what your levels are. Your doctor should be able to run the test, or you can order your blood test online (in the US) through a place like UltaLab Tests. It’s fairly inexpensive (~$28 for men, total testosterone), so you may want to consider testing before you start any Lifehacks and then test again after a few months to see if it’s working for you.
Timing of testing:
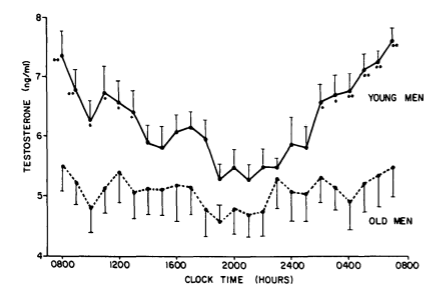
Testosterone levels fluctuate throughout the day in a circadian rhythm, with the highest levels in the morning.[ref] If you are going to test your testosterone periodically to see how it changes, always get the blood drawn around the same time
Eating before testing:
Testosterone levels drop by up to 25% after eating carbohydrates.[ref] This is a very significant fluctuation, and if you want to be able to compare test results, be sure that you either always get your blood work done first thing in the morning after fasting all night – or get it done at the same time after a meal.
Seasonal changes:
There is also a small seasonal effect on average testosterone levels. Testosterone levels tend to be highest in the fall and dip to a low in the spring.[ref][ref] Not all studies show this seasonal change, so it may vary by population group or latitude.
Avoiding microplastics:
This is a harder topic to tackle due to the massive prevalence of microplastics in food, water, and even the air. However, the animal studies on microplastics make a compelling case that the presence of microplastics in the body is detrimental to testosterone production.
Consider tackling the microplastics problem with a two-pronged approach:
- Reducing the amount of plastic you’re exposed to
- Reduce the amount you absorb into the bloodstream
Reducing plastic exposure:
This is a huge topic, so I’m just going to hit a few highlights here. For more ideas, check out this article on why microplastics are likely a big health problem.
- As plastic food packaging breaks down, it gives off tiny plastic particles. Storing food in glass instead of plastic will help.
- Drinking from plastic cups (styrofoam, plastic bottles, paper coffee cups lined with a thin layer of plastic) imparts microplastics into your drink, especially with hot drinks. Take a coffee mug with you if you are going to get a hot beverage while you’re out, or go with a refillable stainless steel cup or tumbler with you for cold drinks. Even just rinsing out the to-go coffee cup with cut microplastic dust on the cup by half.[ref]
- Household dust contains a lot of microplastic from fabric fibers from your furniture and clothes. Regularly dusting and vacuuming will reduce your exposure.
- Tap water often contains microplastics. Filter your water using a good water filter, such as reverse osmosis.
Reduce the amount of microplastic you absorb into the bloodstream
Only a portion of the microplastics that we get from food or drinks will end up being absorbed in the intestines. It turns out that having an optimal gut mucosal barrier will help to prevent microplastics from being absorbed. Avoiding emulsifiers and surfactants in processed foods will help with optimal mucosal barrier thickness. Here’s more on the gut mucosal barrier, genetic interactions, and how to prevent it from thinning.
Avoiding endocrine disruptors:
BPA:
Environmental exposure to BPA and phthalates is associated with increased SHBG levels and decreased testosterone (total and free) in children and teens.[ref] BPA and BPF are found in plastics, including some plastic bottles and food wrappers.
Related article: BPA and your genes
Phthalates:
Another study looked at phthalate levels in a larger group and found that increasing phthalate levels were associated with decreased testosterone levels for boys and men and women ages 40-60.[ref] Phthalates are used as plasticizers, such as in vinyl, and also in artificial fragrances, such as in laundry detergents and air fresheners. Switching to fragrance-free detergents, lotions, and personal care products can help reduce your phthalate exposure. See the related article for more tips.
Related article: Detoxifying Phthalates: Genes and Diet
Avoiding anti-androgens:
Fried fast food:
It turns out that fried foods may not be all that good for you… who knew? A study in animals showed that consuming oxidized frying oil (compared to fresh soybean oil) decreased testosterone concentrations significantly. It also decreased sperm motility.[ref]
What about soy?
Contrary to the soy boy memes, a review of 41 studies from 2010-2020 found that there was no statistical effect from soy protein or isoflavones on total testosterone or free testosterone levels.[ref]
Lifestyle factors that affect SHBG levels:
In addition to genetic variants, certain lifestyle factors can also impact SHBG levels. Avoiding these may help out if you have a genetic propensity towards higher SHBG/lower free testosterone.
Intense physical exercise:
Studies show that intense physical exercise causes an increase in SHBG and a subsequent decrease in free testosterone.[ref] Regular exercise, like lifting weights, causes an immediate transient increase in testosterone for a short period of time. Again, the timing of exercise could affect your testosterone levels when testing it (e.g. don’t stop at the gym before getting the blood test).
Alcohol:
Chronic alcohol consumption (>20g/day) also increases SHBG and decreases testosterone.[ref]
High protein diet:
A diet high in protein is associated with lower SHBG levels.[ref] If your SHBG levels tend to the high side due to genetics, you could try shifting to a diet higher in protein.
Maximizing testosterone production:
Related Articles and Topics:
HPA Axis Dysfunction: Understanding Cortisol and Genetic Interactions
References:
Bogaert, Veerle, et al. “Heritability of Blood Concentrations of Sex-Steroids in Relation to Body Composition in Young Adult Male Siblings.” Clinical Endocrinology, vol. 69, no. 1, July 2008, pp. 129–35. PubMed, https://doi.org/10.1111/j.1365-2265.2008.03173.x.
El Tarhouny, S. A., et al. “Study of Sex Hormone-Binding Globulin Gene Polymorphism and Risk of Type 2 Diabetes Mellitus in Egyptian Men.” The West Indian Medical Journal, vol. 64, no. 4, Sept. 2015, pp. 338–43. PubMed, https://doi.org/10.7727/wimj.2014.088.
Ferguson, Kelly K., et al. “Prenatal and Peripubertal Phthalates and Bisphenol-A in Relation to Sex Hormones and Puberty in Boys.” Reproductive Toxicology (Elmsford, N.Y.), vol. 0, Aug. 2014, pp. 70–76. PubMed Central, https://doi.org/10.1016/j.reprotox.2014.06.002.
Fui, Mark Ng Tang, et al. “Lowered Testosterone in Male Obesity: Mechanisms, Morbidity and Management.” Asian Journal of Andrology, vol. 16, no. 2, 2014, pp. 223–31. PubMed Central, https://doi.org/10.4103/1008-682X.122365.
Grigorova, Marina, Margus Punab, Kristo Ausmees, et al. “FSHB Promoter Polymorphism within Evolutionary Conserved Element Is Associated with Serum FSH Level in Men.” Human Reproduction (Oxford, England), vol. 23, no. 9, Sept. 2008, pp. 2160–66. PubMed Central, https://doi.org/10.1093/humrep/den216.
Grigorova, Marina, Margus Punab, Olev Poolamets, Mart Adler, et al. “Genetics of Sex Hormone-Binding Globulin and Testosterone Levels in Fertile and Infertile Men of Reproductive Age.” Journal of the Endocrine Society, vol. 1, no. 6, Apr. 2017, pp. 560–76. PubMed Central, https://doi.org/10.1210/js.2017-00050.
Grigorova, Marina, Margus Punab, Olev Poolamets, Piret Kelgo, et al. “Increased Prevalance of the −211 T Allele of Follicle Stimulating Hormone (FSH) β Subunit Promoter Polymorphism and Lower Serum FSH in Infertile Men.” The Journal of Clinical Endocrinology and Metabolism, vol. 95, no. 1, Jan. 2010, pp. 100–08. PubMed Central, https://doi.org/10.1210/jc.2009-1010.
Jin, Guangfu, et al. “Genome-Wide Association Study Identifies a New Locus JMJD1C at 10q21 That May Influence Serum Androgen Levels in Men.” Human Molecular Genetics, vol. 21, no. 23, Dec. 2012, pp. 5222–28. PubMed, https://doi.org/10.1093/hmg/dds361.
Leinonen, Jaakko T., et al. “LIN28B Affects Gene Expression at the Hypothalamic-Pituitary Axis and Serum Testosterone Levels.” Scientific Reports, vol. 9, Dec. 2019, p. 18060. PubMed Central, https://doi.org/10.1038/s41598-019-54475-6.
Lerchbaum, Elisabeth, et al. “Hyperandrogenemia in Polycystic Ovary Syndrome: Exploration of the Role of Free Testosterone and Androstenedione in Metabolic Phenotype.” PLoS ONE, vol. 9, no. 10, Oct. 2014, p. e108263. PubMed Central, https://doi.org/10.1371/journal.pone.0108263.
Lopresti, Adrian L., et al. “A Randomized, Double-Blind, Placebo-Controlled, Crossover Study Examining the Hormonal and Vitality Effects of Ashwagandha ( Withania Somnifera) in Aging, Overweight Males.” American Journal of Men’s Health, vol. 13, no. 2, Apr. 2019, p. 1557988319835985. PubMed, https://doi.org/10.1177/1557988319835985.
Meeker, John D., and Kelly K. Ferguson. “Urinary Phthalate Metabolites Are Associated With Decreased Serum Testosterone in Men, Women, and Children From NHANES 2011–2012.” The Journal of Clinical Endocrinology and Metabolism, vol. 99, no. 11, Nov. 2014, pp. 4346–52. PubMed Central, https://doi.org/10.1210/jc.2014-2555.
Mohammadi-Shemirani, Pedrum, et al. “Effects of Lifelong Testosterone Exposure on Health and Disease Using Mendelian Randomization.” ELife, vol. 9, p. e58914. PubMed Central, https://doi.org/10.7554/eLife.58914. Accessed 19 May 2022.
Ohlsson, Claes, et al. “Genetic Determinants of Serum Testosterone Concentrations in Men.” PLOS Genetics, vol. 7, no. 10, Oct. 2011, p. e1002313. PLoS Journals, https://doi.org/10.1371/journal.pgen.1002313.
Oskui, Peyman Mesbah, et al. “Testosterone and the Cardiovascular System: A Comprehensive Review of the Clinical Literature.” Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease, vol. 2, no. 6, Dec. 2013, p. e000272. PubMed Central, https://doi.org/10.1161/JAHA.113.000272.
Panizzon, Matthew S., et al. “Genetic and Environmental Influences of Daily and Intra-Individual Variation in Testosterone Levels in Middle-Aged Men.” Psychoneuroendocrinology, vol. 38, no. 10, Oct. 2013, pp. 2163–72. PubMed Central, https://doi.org/10.1016/j.psyneuen.2013.04.003.
Roosenboom, Jasmien, et al. “SNPs Associated With Testosterone Levels Influence Human Facial Morphology.” Frontiers in Genetics, vol. 9, Oct. 2018, p. 497. PubMed Central, https://doi.org/10.3389/fgene.2018.00497.
Ruth, Katherine S., et al. “Using Human Genetics to Understand the Disease Impacts of Testosterone in Men and Women.” Nature Medicine, vol. 26, no. 2, Feb. 2020, pp. 252–58. PubMed Central, https://doi.org/10.1038/s41591-020-0751-5.
Sarkar, Monika, et al. “Testosterone Levels in Pre-Menopausal Women Are Associated With Nonalcoholic Fatty Liver Disease in Midlife.” The American Journal of Gastroenterology, vol. 112, no. 5, May 2017, pp. 755–62. PubMed Central, https://doi.org/10.1038/ajg.2017.44.
Wankhede, Sachin, et al. “Examining the Effect of Withania Somnifera Supplementation on Muscle Strength and Recovery: A Randomized Controlled Trial.” Journal of the International Society of Sports Nutrition, vol. 12, 2015, p. 43. PubMed, https://doi.org/10.1186/s12970-015-0104-9.
Originally published 10/2018. Updated 3/2020.