Key takeaways:
- IL-15 is a proinflammatory cytokine that is essential for the development, survival, and activation of NK cells and CD8+ T cells, which are important in the immune response to pathogens and cancer.
- Dysregulated or chronically high IL-15 signaling contributes to several autoimmune diseases, osteoarthritis pain, and certain blood cancers, making “too much IL-15” a problem in some contexts. Low IL-15 may contribute to a blunted immune response to cancer or persistent infections.
- Genetic variants in IL15 and IL15RA influence muscle strength gains from exercise as well as risks for celiac disease, leukemia, and osteoarthritis.
- Lifestyle and supplement factors—including sleep/circadian alignment, exercise, vitamin D, zinc, probiotics, beta-alanine, butyrate, and colostrum—can modulate IL-15 signaling up or down, which may be helpful depending on whether infection, autoimmunity, or cancer is the primary concern.
Interleukin-15 (IL-15) and Its Genetic Variants
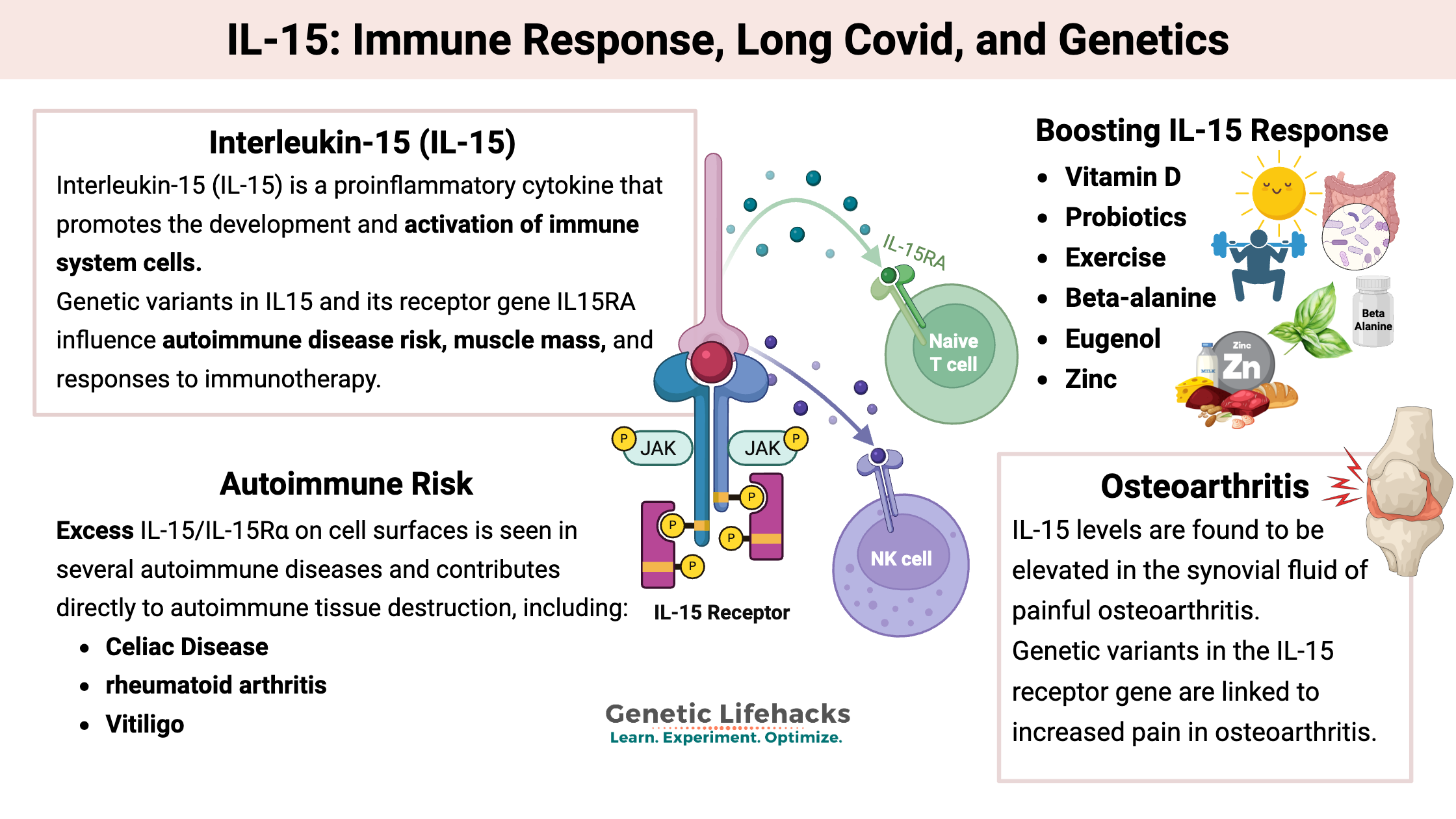
Interleukin-15 (IL-15) is a proinflammatory cytokine that promotes the development and activation of immune system cells, especially natural killer (NK) cells and CD8+ T cells. These immune cells, called lymphocytes, are central to the immune response to infections and cancer. This immune response and IL-15 are also implicated in autoimmune diseases.
IL-15 is essential for the growth, maintenance, and function of NK cells and long-lived T cells. It also enhances immune responses against viral and bacterial infections. While completely necessary for fighting cancer and pathogens, IL-15 overproduction is linked to autoimmune disorders and other chronic inflammatory conditions. Its expression is tightly regulated at multiple levels to prevent uncontrolled inflammation and autoimmunity.
Genetic variants in IL15 and its receptor gene IL15RA influence autoimmune disease risk, muscle mass, and responses to immunotherapy.
Background on T cells and natural killer cells:
T cells are a type of white blood cell, also called lymphocytes. They are part of the adaptive immune system.
There are multiple types of T cells, according to the Cleveland Clinic:[ref]
- Cytotoxic T cells, also called CD8+ cells, are the type of T cells that will kill cells infected with viruses or bacteria. They are the ones that kill tumor cells also.
- Helper T cells, which are also called CD4+ cells because they have a CD4 receptor on them, signal to the rest of the immune system that it needs to attack.
- Regulatory T cells, called T regs, help to modulate the immune response and reduce the activity of other T cells, keeping your own healthy cells from being attacked.
Related article: T cell exhaustion, checkpoint genes
Natural killer cells (NK) are another type of lymphocyte that can destroy cancer cells and infected cells. IL-15 can also activate natural killer cells.[ref]
IL-15: Soluble and membrane-bound
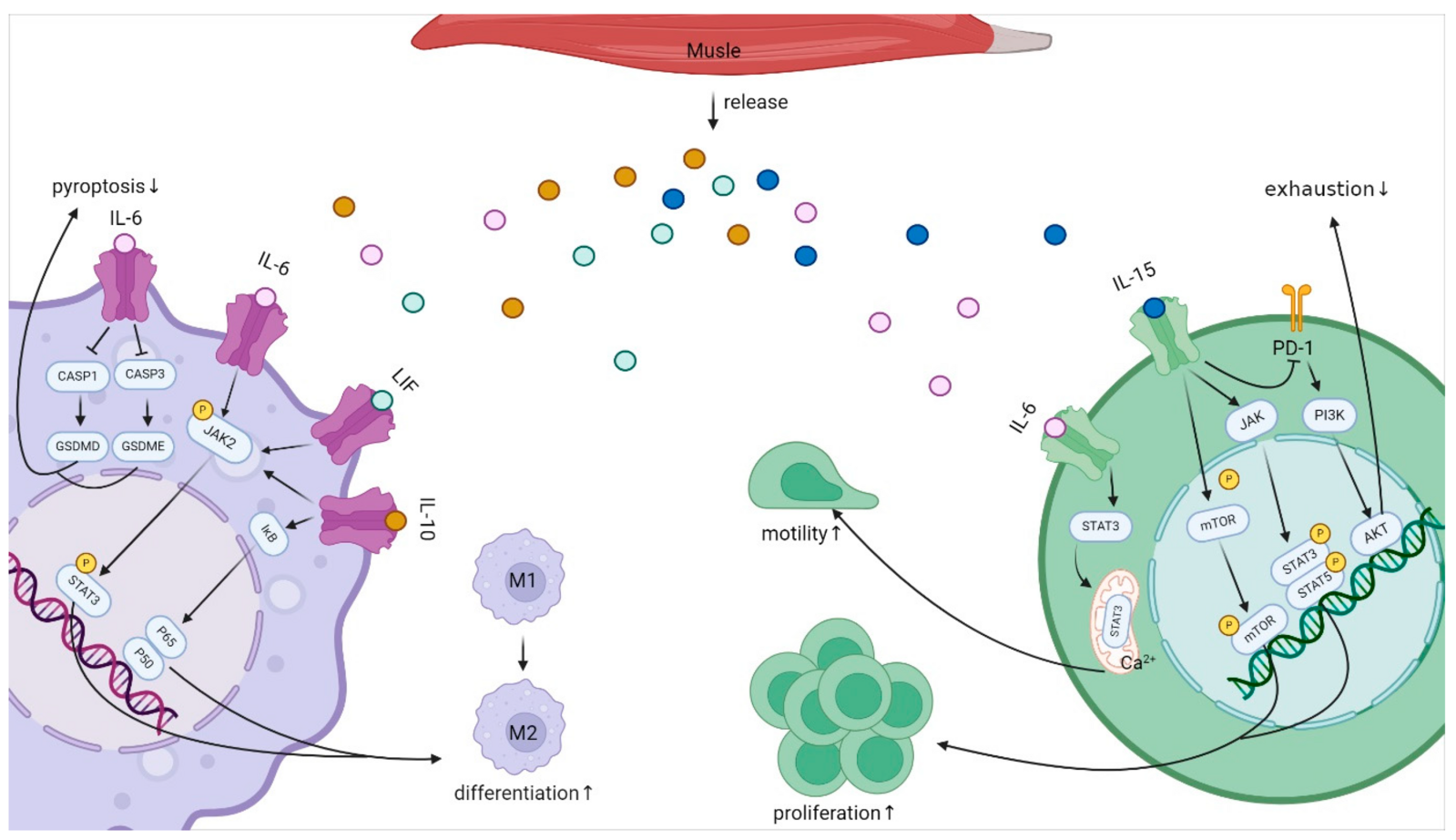
IL-15 is produced by several immune cell types, including monocytes, macrophages, and dendritic cells. It is also produced in the skeletal muscles.
Two forms: IL-15 exists both as a soluble form, which circulates at low levels in the body, and as a membrane-bound form that is either directly on the cell membrane or presented with the IL-15Rα receptor. [ref]
- Membrane-bound: The intercellular form is the primary form. It is retained in the cells and forms a complex with the IL-15 receptor alpha (IL-15Rα) for presentation on the surface of cells. This stable complex on the cell surface allows the persistence of the signal.[ref]
- Soluble: Small amounts of free soluble IL‑15 circulate in the blood, along with low levels of a soluble IL‑15–IL‑15Rα complex..[ref] (We will come back to the complex of IL-15 and IL-15Rα as a cancer and possible long Covid drug.)
Regulation: IL-15 is regulated in multiple ways, balancing excess inflammation with a strong enough immune response.
Autoimmune diseases:
Excess IL‑15/IL‑15Rα on cell surfaces is seen in several autoimmune diseases and contributes directly to autoimmune tissue destruction..[ref]
- In celiac disease, IL-15 is upregulated in the intestinal epithelial cells. Studies show that IL-15 is critical to active celiac disease, and it is required for CD8+ T cells to cause tissue destruction in the intestinal villi. Serum IL-15 levels are also elevated in newly diagnosed celiac patients.[ref][ref] (See genotype report)
- In rheumatoid arthritis, IL‑15 is upregulated and plays an active role in joint inflammation and destruction. [ref][ref]
- Vitiligo is an autoimmune disease that kills the melanocytes that give skin its color. IL-15 plays an active role in the CD8+ T-cell-mediated melanocyte destruction.[ref]
Response to viruses:
IL-15 plays an important role in stimulating T cell and natural killer cell proliferation in response to a viral or bacterial infection.
- Hepatitis C: IL-15 is important in the immune response to hepatitis C viral infections. IL‑15 levels correlate with the ability to clear the hepatitis C virus, but higher levels are also associated with an increased risk of liver fibrosis.[ref]
- Epstein-Barr Virus: In addition to causing mononucleosis in teens and young adults, the Epstein-Barr virus can linger in an inactive state in the body for life. It is implicated as playing a role in autoimmune diseases, such as MS, and can be reactivated at times of immune system impairment. EBV also plays a causal role in a specific type of blood cancer called Burkitt’s lymphoma. Researchers have found that increasing IL-15 may be a way to help boost immunotherapy in patients with EBV-associated malignancies.[ref]
- Severe Covid: Researchers looked in depth at what happens to T cells after a severe SARS-CoV-2 infection. The results showed sustained elevation of IL-15 levels for up to a year post-infection. They also found persistent T cell activation, even months after recovery from the acute Covid symptoms, and signs of T cell exhaustion.[ref]
- Vaccination for Covid: Research shows that the initial Covid mRNA vaccine series increases IL-15 levels by 2-fold immediately following the second injection.[ref]
Long Covid, long spike, or chronic viral infections:
Since IL-15 is integral to CD8+ T cell and natural killer survival and proliferation, it can also be key to whether a viral infection is cleared or allowed to linger. Persistent infections can occur when the immune response isn’t strong enough to completely clear the virus.
- For some people with long Covid, persistent viral replication is found, and signs of T cell exhaustion or persistent activation are noted.[ref][ref][ref][ref][ref]
- A December 2025 study showed that in many people with post-COVID-19 vaccine syndrome (PCVS), the S1 spike protein from the mRNA vaccine persists for more than 8 months and alters immune cell levels.[ref]
- In chronic hepatitis B infections, the virus binds to an IL-15 receptor on natural killer cells and causes NK dysfunction. [ref]
An IL-15 drug, ANKTIVA, is currently being studied in a phase II clinical trial for long Covid. The aim is to boost NK activity to clear out any lingering virus or viral debris.[ref]
Pregnancy and IL-15:
During pregnancy, there is a fine balance in the immune system so that the mother’s body doesn’t attack the fetus as foreign tissue.
IL-15 is found in the uterus and placenta, where it plays an important role in preventing pregnancy loss. IL-15 also promotes NK cells, which are important for vascular remodeling and placental development.
Balance is key: both an excess of IL-15 and a complete deficiency of IL-15 can cause adverse outcomes in pregnancy. Low IL-15 is found in pregnant women with pre-eclampsia.[ref]
Cancer: Boosting the immune response for tumors
IL-15 is essential for CD8+ T cell and natural killer cell responses in cancer. Increasing IL-15 is one area of active research to help the body’s own T cell response to kill off cancer cells in solid tumors. T cells can get exhausted and stop actively reacting to cancer cells, but adding more IL-15 can reverse T cell exhaustion and promote tumor cell death. Additionally, IL-15 can boost natural killer cell immune response.[ref][ref]
However, all cancers are not the same. In blood cancers, such as leukemia and lymphoma, high levels of IL-15 can be detrimental and promote cancer cell growth.[ref]
Access this content:
An active subscription is required to access this content.
Related articles:
T Cell Exhaustion in Long COVID, ME/CFS, and Cancer: Mechanisms and Solutions
Epstein-Barr Virus: Genetic Risks, Reactivation, and Chronic Illnesses
References: