Key takeaways:
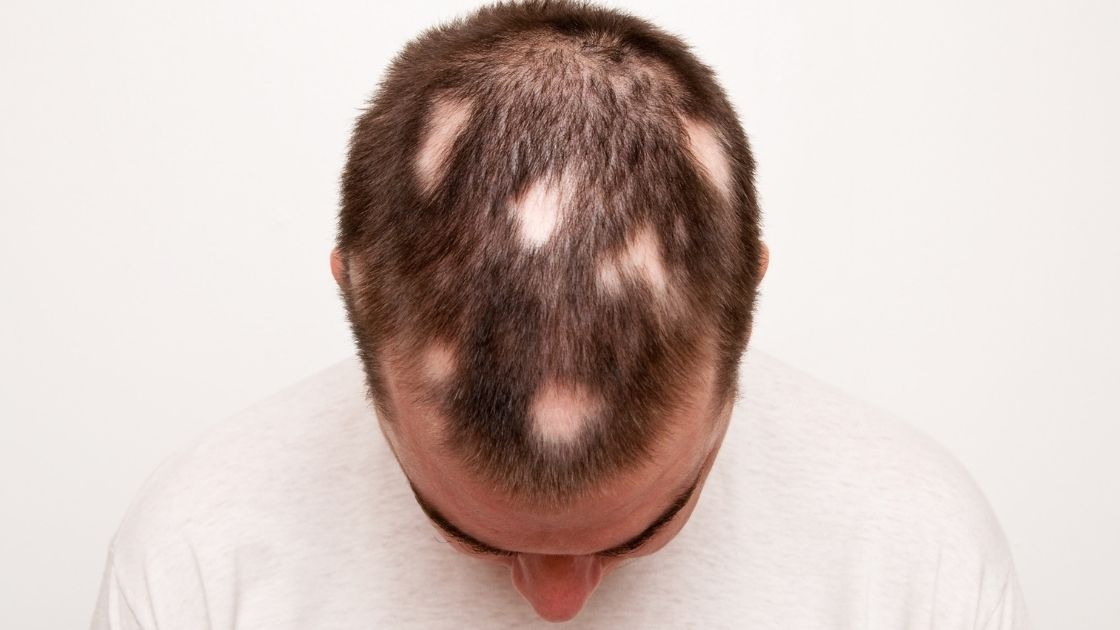
~ Alopecia areata is an autoimmune-like disease that causes sudden and rapid hair loss. Often it causes circular bald patches at random spots on the scalp.
~ Genetic variants related to inflammation and immune response increase the risk of alopecia areata, especially in conjunction with a triggering factor.
~ Natural options are available to fight against alopecia areata, and understanding your genetic variants can help guide you in the pathway to target.
What causes alopecia areata?
Alopecia areata is an autoimmune-like disorder in which the immune system attacks the hair follicle. It is a complex, polygenic, immune-mediated disease that can affect people at any age. The average age of onset is in the 30s, and it occurs at the same rate in both males and females. The lifetime risk for alopecia areata is ~2%, so you are not alone in this! It is the most frequent autoimmune disorder worldwide.[ref][ref][ref]
Coin-sized spots of hair loss are commonly how alopecia areata begins. But alopecia areata can cause:[ref]
- widespread hair loss
- patchy hair loss
- loss of eyelashes
- brittle nails
- bald patches in beards
Hair loss is due to the infiltration of white blood cells into the hair follicle, damaging the cells and causing hair loss.
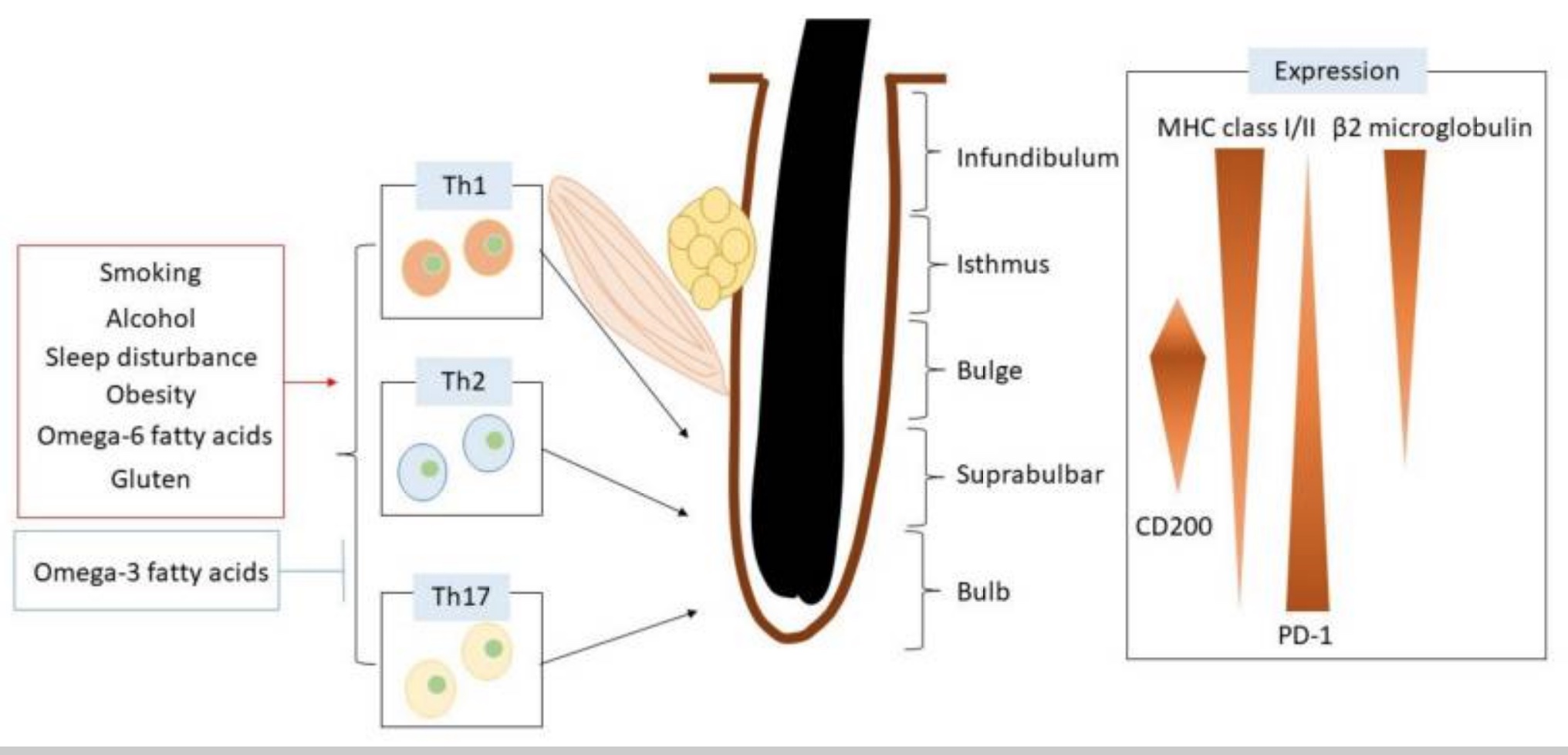
Normally, hair follicles are ‘immune privleged’. This term means that the immune system doesn’t see the hair follicle. It is both an active and passive mechanism protecting the hair follicle from an immune response. The hair follicle cells present signals (HLA class I and class II molecules) that tell the immune system everything is ok. T regulatory cells (Tregs) are also present to keep the immune response at bay.
Immune privilege is a state that protects certain tissues in the body from being challenged by the immune system. For example, tissues in the eye, testes, and placenta have ‘immune privilege’.
Researchers theorized that hair follicles need immune privilege during the pigment activation and hair shaft production phases. The activation of melanocytes for pigmentation can trigger immune cells. Plus, for most mammals, hair maintenance is a survival factor that needs to be protected even when there is a wound. Thus, researchers theorize that the immune system needs to be modulated to keep it from being overly activated in the hair follicles (and likely in the feather follicles in birds).[ref]
In the area of the hair follicles, cells generate immunosuppressants during the period in which hair is actively growing, which is called the anagen phase. The cells produce molecules such as TGF-B1 and MIF, which suppress natural killer cells.[ref]
In alopecia areata, immune privilege goes awry.
- T cells (white blood cells) and natural killer cells infiltrate the hair follicle.
- Mast cells also are activated and give off inflammatory payloads.[ref]
- Inflammatory cytokine levels elevate. Specifically, IL-2, TNF-alpha, IL-12, and IL-17 are higher in alopecia areata patients.[ref][ref]
- Excessive interferon-gamma production, such as during a viral infection, can also upregulate T cells in the hair follicle.[ref]
Triggers of alopecia areata:
Alopecia areata occurs more often in people with depression, anxiety, Hashimoto’s, lupus, vitiligo, psoriasis, RA, and inflammatory bowel diseases. What do all of these conditions have in common? Overactivation of the immune system.
Most people (more than 80%) will spontaneously regrow hair within a year of the onset of alopecia areata. But, like many immune system-related diseases, relapses are common…
There are two pathways that researchers think can lead to hair loss:[ref]
- Autoimmune alopecia areata is induced by autoreactive CD8+ T cells that respond to hair follicle-associated autoantigens. This response is coupled with insufficient T regulatory activity to counteract the autoimmune response.
- Alternatively, some cases may not truly be an autoimmune disorder. Researchers also think that alopecia areata can be triggered by excessive natural killer cells or mast cell activities. It could be coupled with or caused by dysbiosis of the hair follicle microbiome.[ref]
Both can trigger the collapse of the hair follicle immune privilege, leading to infiltration by T cells, a type of white blood cell, and apoptosis of hair follicle cells.
Interferon-gamma: During viral infections, interferon-gamma is elevated dramatically. For example, alopecia areata was triggered in a few individuals by the swine flu.[ref]
Th17 shift: T helper 17 cells are a subset of T cells that produce inflammatory cytokines (IL-17A and IL17F). Th17 cells are implicated in causing several different autoimmune diseases, such as RA, lupus, and psoriasis. Researchers have also found Th17 cells in the affected hair follicles of people with alopecia areata.[ref]
Lifestyle factors: Environmental and dietary factors influence the risk of alopecia areata. These include:[ref]
- Smoking activates Th17 cells and increases IL-17 production.
- Sleep disorders cause a 300% increase in the risk for alopecia areata.
- Obesity causes a 15% increase in the risk of alopecia areata.
- Alcohol at normal consumption levels is actually associated with a lower risk of AA.
Here’s a graphical overview of what is impacting the hair follicle.
Is alopecia areata hereditary?
Alopecia areata has a genetic component. Genetic variants related to elevated immune response and susceptibility to autoimmune diseases increase the relative risk of AA. But genes alone do not cause AA. Instead, it is a combination of environmental triggers and genetic susceptibility.
Types of alopecia areata
There are multiple types of AA, depending on the extent and location of hair loss:[ref]
- Patchy alopecia areata: multiple patches of hair loss on the scalp.
- Alopecia totalis: total or near-total loss of hair, but only on the scalp.
- Alopecia universalis: total to near-total hair loss everywhere – all body hair is affected.
- Alopecia incognita: diffuse total hair loss across the scalp with short, miniaturized regrowing hairs.
- Ophiasis: hair loss in a band-like shape around the head.
- Sisaipho: extensive alopecia except around the periphery of the scalp.
- Marie Antoinette syndrome (also called canities subita): sudden, diffuse alopecia with very sudden “overnight” greying.
- Alopecia barbae: bald spots in your beard
Covid-19 vaccine and alopecia areata:
Alopecia areata has been reported in conjunction with the SARS-CoV-2 vaccines. Here are some of the case reports published by physicians.
- A case series was written about 9 people with AA following SARS-CoV-2 vaccination. None of the people had previously had AA. On average, the patients were seeking treatment for AA about 2 -8 weeks after their second dose of the vaccine.[ref]
- Another case report was on an 80-year-old who had hair (though with some hair loss) before the vaccine. A week after the Pfizer vaccine, he had hair loss and patchy beard loss. After the second vaccine, he had total hair loss (alopecia areata totalis).[ref]
- A case series showed rapid hair loss in people who had previously had AA. They all had rapid hair loss within a week of vaccination (Pfizer and AstraZeneca).
Alopecia Areata Genotype Report
Related Articles and Topics:
TNF-alpha: Inflammation, Chronic Diseases, and Genetic Susceptibility
Specialized Pro-resolving Mediators (SPMs): The Resolution of Inflammation
References:
Aytekin, Nesrin, et al. “Investigation of Interleukin-12, Interleukin-17 and Interleukin-23 Receptor Gene Polymorphisms in Alopecia Areata.” The Journal of International Medical Research, vol. 43, no. 4, Aug. 2015, pp. 526–34. PubMed, https://doi.org/10.1177/0300060514549784.
Bertolini, Marta, et al. “Hair Follicle Immune Privilege and Its Collapse in Alopecia Areata.” Experimental Dermatology, vol. 29, no. 8, Aug. 2020, pp. 703–25. DOI.org (Crossref), https://doi.org/10.1111/exd.14155.
Bin Huraib, Ghaleb, et al. “The Protein Tyrosine Phosphatase Nonreceptor 22 (PTPN22) R620W Functional Polymorphism in Psoriasis.” Clinical Medicine Insights. Arthritis and Musculoskeletal Disorders, vol. 11, 2018, p. 1179544117751434. PubMed, https://doi.org/10.1177/1179544117751434.
Celik, Sumeyya Deniz, and Omer Ates. “Genetic Analysis of Interleukin 18 Gene Polymorphisms in Alopecia Areata.” Journal of Clinical Laboratory Analysis, vol. 32, no. 5, June 2018, p. e22386. PubMed, https://doi.org/10.1002/jcla.22386.
Cristina, Cantú-Salinas, et al. “Tumor Necrosis Factor Alpha Promoter-308G/A Polymorphism in Mexican Patients with Patchy Alopecia Areata.” International Journal of Dermatology, vol. 51, no. 5, May 2012, pp. 571–75. PubMed, https://doi.org/10.1111/j.1365-4632.2011.05291.x.
Ding, Cheng, et al. “TNF-α Gene Promoter Polymorphisms Contribute to Periodontitis Susceptibility: Evidence from 46 Studies.” Journal of Clinical Periodontology, vol. 41, no. 8, Aug. 2014, pp. 748–59. PubMed, https://doi.org/10.1111/jcpe.12279.
Eskandari-Nasab, Ebrahim, et al. “Meta-Analysis of Risk Association Between Interleukin-17A and F Gene Polymorphisms and Inflammatory Diseases.” Journal of Interferon & Cytokine Research: The Official Journal of the International Society for Interferon and Cytokine Research, vol. 37, no. 4, Apr. 2017, pp. 165–74. PubMed, https://doi.org/10.1089/jir.2016.0088.
Fathima, Nusrath, et al. “Association and Gene-Gene Interaction Analyses for Polymorphic Variants in CTLA-4 and FOXP3 Genes: Role in Susceptibility to Autoimmune Thyroid Disease.” Endocrine, vol. 64, no. 3, June 2019, pp. 591–604. PubMed, https://doi.org/10.1007/s12020-019-01859-3.
Fricke, Alexandra C. Villasante, and Mariya Miteva. “Epidemiology and Burden of Alopecia Areata: A Systematic Review.” Clinical, Cosmetic and Investigational Dermatology, vol. 8, 2015, p. 397. www.ncbi.nlm.nih.gov, https://doi.org/10.2147/CCID.S53985.
Gorman, Jennifer D., et al. “Particular HLA-DRB1 Shared Epitope Genotypes Are Strongly Associated with Rheumatoid Vasculitis.” Arthritis and Rheumatism, vol. 50, no. 11, Nov. 2004, pp. 3476–84. PubMed, https://doi.org/10.1002/art.20588.
Hair Loss Types: Alopecia Areata Signs and Symptoms. https://www.aad.org/public/diseases/hair-loss/types/alopecia/symptoms. Accessed 3 Mar. 2022.
Huraib, Ghaleb Bin, et al. “Association of Functional Polymorphism in Protein Tyrosine Phosphatase Nonreceptor 22 (PTPN22) Gene with Vitiligo.” Biomarker Insights, vol. 15, Jan. 2020, p. 1177271920903038. PubMed Central, https://doi.org/10.1177/1177271920903038.
Ismail, Nader Ali, et al. “Association of Rs231775 Genetic Variant of Cytotoxic T-Lymphocyte Associated Protein 4 with Alopecia Areata Disease in Males: A Case–Control Study.” Immunological Investigations, vol. 50, no. 8, Nov. 2021, pp. 977–86. Taylor and Francis+NEJM, https://doi.org/10.1080/08820139.2020.1796700.
Ji, Conghua, et al. “HLA-DRB1 Polymorphisms and Alopecia Areata Disease Risk: A Systematic Review and Meta-Analysis.” Medicine, vol. 97, no. 32, Aug. 2018, p. e11790. PubMed, https://doi.org/10.1097/MD.0000000000011790.
Lew, Bark-Lynn, et al. “Association between IL17A/IL17RA Gene Polymorphisms and Susceptibility to Alopecia Areata in the Korean Population.” Annals of Dermatology, vol. 24, no. 1, Feb. 2012, p. 61. www.ncbi.nlm.nih.gov, https://doi.org/10.5021/ad.2012.24.1.61.
Li, Fang, et al. “Association of CTLA-4 Polymorphisms with Increased Risks of Myasthenia Gravis.” Annals of Human Genetics, vol. 82, no. 6, Nov. 2018, pp. 358–69. PubMed, https://doi.org/10.1111/ahg.12262.
Majumder, Poulami, et al. “Association of Tumor Necrosis Factor-α (TNF-α) Gene Promoter Polymorphisms with Aggressive and Chronic Periodontitis in the Eastern Indian Population.” Bioscience Reports, vol. 38, no. 4, Aug. 2018, p. BSR20171212. PubMed, https://doi.org/10.1042/BSR20171212.
Marwa, Ouled Salah, et al. “Association of IL17A and IL17F Genes with Rheumatoid Arthritis Disease and the Impact of Genetic Polymorphisms on Response to Treatment.” Immunology Letters, vol. 183, Mar. 2017, pp. 24–36. PubMed, https://doi.org/10.1016/j.imlet.2017.01.013.
May Lee, Marco, et al. “Alopecia Areata Following COVID ‐19 Vaccination: Vaccine‐induced Autoimmunity?” International Journal of Dermatology, Feb. 2022, p. ijd.16113. DOI.org (Crossref), https://doi.org/10.1111/ijd.16113.
Mewes, Caspar, et al. “The CTLA-4 Rs231775 GG Genotype Is Associated with Favorable 90-Day Survival in Caucasian Patients with Sepsis.” Scientific Reports, vol. 8, no. 1, Oct. 2018, p. 15140. PubMed, https://doi.org/10.1038/s41598-018-33246-9.
Minokawa, Yoko, et al. “Lifestyle Factors Involved in the Pathogenesis of Alopecia Areata.” International Journal of Molecular Sciences, vol. 23, no. 3, Feb. 2022. www.ncbi.nlm.nih.gov, https://doi.org/10.3390/ijms23031038.
Oh, Eun Hye, et al. “Rare Variants of Putative Candidate Genes Associated With Sporadic Meniere’s Disease in East Asian Population.” Frontiers in Neurology, vol. 10, Jan. 2020, p. 1424. PubMed Central, https://doi.org/10.3389/fneur.2019.01424.
Pabalan, Noel, et al. “Association of the Protein Tyrosine Phosphatase Non-Receptor 22 Polymorphism (PTPN22) with Endometriosis: A Meta-Analysis.” Einstein (Sao Paulo, Brazil), vol. 15, no. 1, Mar. 2017, pp. 105–11. PubMed, https://doi.org/10.1590/S1679-45082017RW3827.
Petukhova, Lynn, et al. “Genome-Wide Association Study in Alopecia Areata Implicates Both Innate and Adaptive Immunity.” Nature, vol. 466, no. 7302, July 2010, p. 113. www.ncbi.nlm.nih.gov, https://doi.org/10.1038/nature09114.
Pratt, C. Herbert, et al. “Alopecia Areata.” Nature Reviews. Disease Primers, vol. 3, Mar. 2017, p. 17011. www.ncbi.nlm.nih.gov, https://doi.org/10.1038/nrdp.2017.11.
Rajabi, Fateme, et al. “Macrophage Migration Inhibitory Factor Polymorphism (Rs755622) in Alopecia Areata: A Possible Role in Disease Prevention.” Archives of Dermatological Research, vol. 311, no. 8, Oct. 2019, pp. 589–94. PubMed, https://doi.org/10.1007/s00403-019-01934-9.
Salinas-Santander, Mauricio, et al. “Association between PTPN22 C1858T Polymorphism and Alopecia Areata Risk.” Experimental and Therapeutic Medicine, vol. 10, no. 5, Nov. 2015, pp. 1953–58. PubMed, https://doi.org/10.3892/etm.2015.2728.
Scollan, Margaret E., et al. “Alopecia Areata after SARS-CoV-2 Vaccination.” JAAD Case Reports, vol. 20, Feb. 2022, p. 1. www.ncbi.nlm.nih.gov, https://doi.org/10.1016/j.jdcr.2021.11.023.
Tanemura, Atsushi, et al. “Alopecia Areata: Infiltration of Th17 Cells in the Dermis, Particularly around Hair Follicles.” Dermatology (Basel, Switzerland), vol. 226, no. 4, 2013, pp. 333–36. PubMed, https://doi.org/10.1159/000350933.
Thompson, Susan D., et al. “The Susceptibility Loci Juvenile Idiopathic Arthritis Shares with Other Autoimmune Diseases Extend to PTPN2, COG6, and ANGPT1.” Arthritis and Rheumatism, vol. 62, no. 11, Nov. 2010, pp. 3265–76. PubMed, https://doi.org/10.1002/art.27688.
Traylor, Matthew, et al. “Genetic Associations with Radiological Damage in Rheumatoid Arthritis: Meta-Analysis of Seven Genome-Wide Association Studies of 2,775 Cases.” PloS One, vol. 14, no. 10, 2019, p. e0223246. PubMed, https://doi.org/10.1371/journal.pone.0223246.
Tu, Yaqin, et al. “Association between Rs3087243 and Rs231775 Polymorphism within the Cytotoxic T-Lymphocyte Antigen 4 Gene and Graves’ Disease: A Case/Control Study Combined with Meta-Analyses.” Oncotarget, vol. 8, no. 66, Nov. 2017, pp. 110614–24. PubMed Central, https://doi.org/10.18632/oncotarget.22702.
Waśkiel-Burnat, Anna, et al. “The Role of Serum Th1, Th2, and Th17 Cytokines in Patients with Alopecia Areata: Clinical Implications.” Cells, vol. 10, no. 12, Dec. 2021. www.ncbi.nlm.nih.gov, https://doi.org/10.3390/cells10123397.
Yan, Ni, et al. “Association of Interleukin-17A and -17F Gene Single-Nucleotide Polymorphisms with Autoimmune Thyroid Diseases.” Autoimmunity, vol. 45, no. 7, Nov. 2012, pp. 533–39. PubMed, https://doi.org/10.3109/08916934.2012.702814.
Żeberkiewicz, Marta, et al. “Immunology of Alopecia Areata.” Central-European Journal of Immunology, vol. 45, no. 3, 2020, p. 325. www.ncbi.nlm.nih.gov, https://doi.org/10.5114/ceji.2020.101264.
Zhang, Peng, et al. “Tumor Necrosis Factor-Alpha Gene Polymorphisms and Susceptibility to Ischemic Heart Disease.” Medicine, vol. 96, no. 14, Apr. 2017, p. e6569. PubMed Central, https://doi.org/10.1097/MD.0000000000006569.
Zhebrun, Daria, et al. “Association of PTPN22 1858T/T Genotype with Type 1 Diabetes, Graves’ Disease but Not with Rheumatoid Arthritis in Russian Population.” Aging (Albany NY), vol. 3, no. 4, Apr. 2011, pp. 368–73. PubMed Central, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3117451/.
Debbie Moon is the founder of Genetic Lifehacks. Fascinated by the connections between genes, diet, and health, her goal is to help you understand how to apply genetics to your diet and lifestyle decisions. Debbie has a BS in engineering from Colorado School of Mines and an MSc in biological sciences from Clemson University. Debbie combines an engineering mindset with a biological systems approach to help you understand how genetic differences impact your optimal health.

