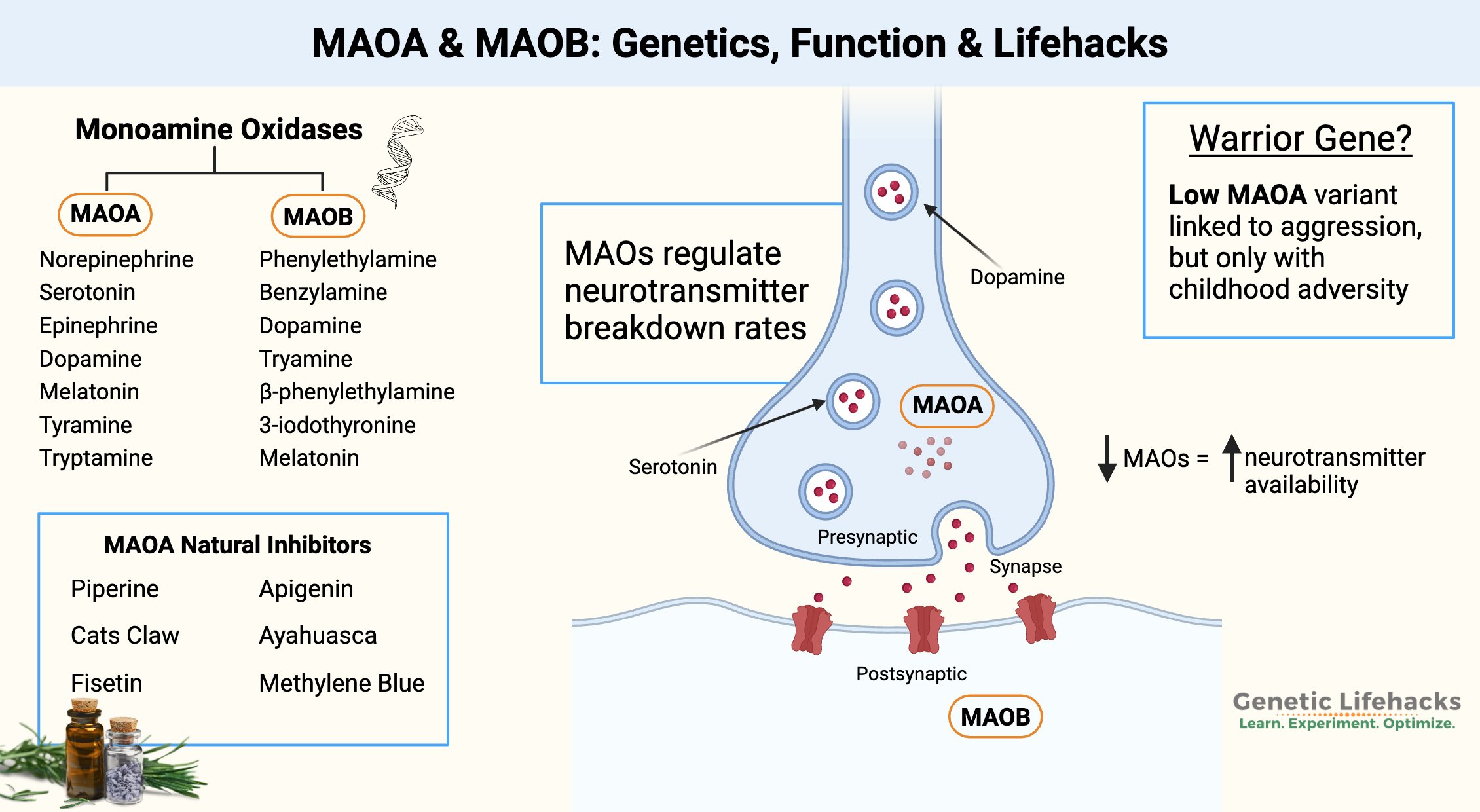
Key takeaways:
~ Monoamine oxidase (MAO) enzymes break down neurotransmitters, regulating the levels of dopamine, serotonin, and more.
~ Higher or lower MAO enzyme levels can affect mood by altering neurotransmitter levels.
~ Genetic variants in the MAOA and MAOB genes can increase the risk of mood disorders or aggression, under some circumstances.
~ There are natural supplements that affect MAO-A and MAO-B levels.
What is the MAO enzyme?
MAO, or monoamine oxidase, is an enzyme that breaks down neurotransmitters. There are two slightly different forms of MAO, MAO-A and MAO-B.
Specifically, monoamine oxidase (MAO) enzymes break down monoamine neurotransmitters – including serotonin, dopamine, and norepinephrine.[ref][ref]
The two enzymes differ a bit in their affinity for different monoamines.
- MAO-A preferentially breaks down norepinephrine, serotonin, epinephrine (adrenaline), melatonin, dopamine, kynuramine, tyramine, and tryptamine.
- MAO-B breaks down dopamine, 3-iodothyronamine, β– Phenylethylamine, kynuramine, and tyramine.
In addition to their roles in neurotransmitter metabolism, MAOs are also involved in the metabolism of certain drugs and biogenic amines. (More on this below in the Parkinson’s section.)
The monoamine oxidases are produced by cells in the brain, as well as throughout the body.
There are a couple of differences in the tissue distribution of the MAOs. MAO-A is most abundant in the heart muscle, platelets, gastrointestinal tract, and specific neurons in the brain.
In the brain, MAO-B is primarily found on the mitochondrial membranes in the astrocytes and specific types of neurons. MAO-B is also found in the heart, intestines, kidneys, and blood vessels.[ref][ref][ref]
MAOs in the Brain:
Neurotransmitter levels are tightly controlled in the brain. You want everything in balance, with neurons firing appropriately.
Neurons release neurotransmitters into the synapse — the area between the terminal of one neurotransmitter and the receptors on the dendrites for the next neuron. Neurotransmitters are the chemical signals that pass the message from one neuron to the next.
The MAO enzymes play an essential role in maintaining the appropriate amount of dopamine, serotonin, norepinephrine, and epinephrine. By breaking down the neurotransmitter, MAO prevents too much of the neurotransmitter from hanging around. Dopamine, for example, will cause damage and kill neurons when in excess.
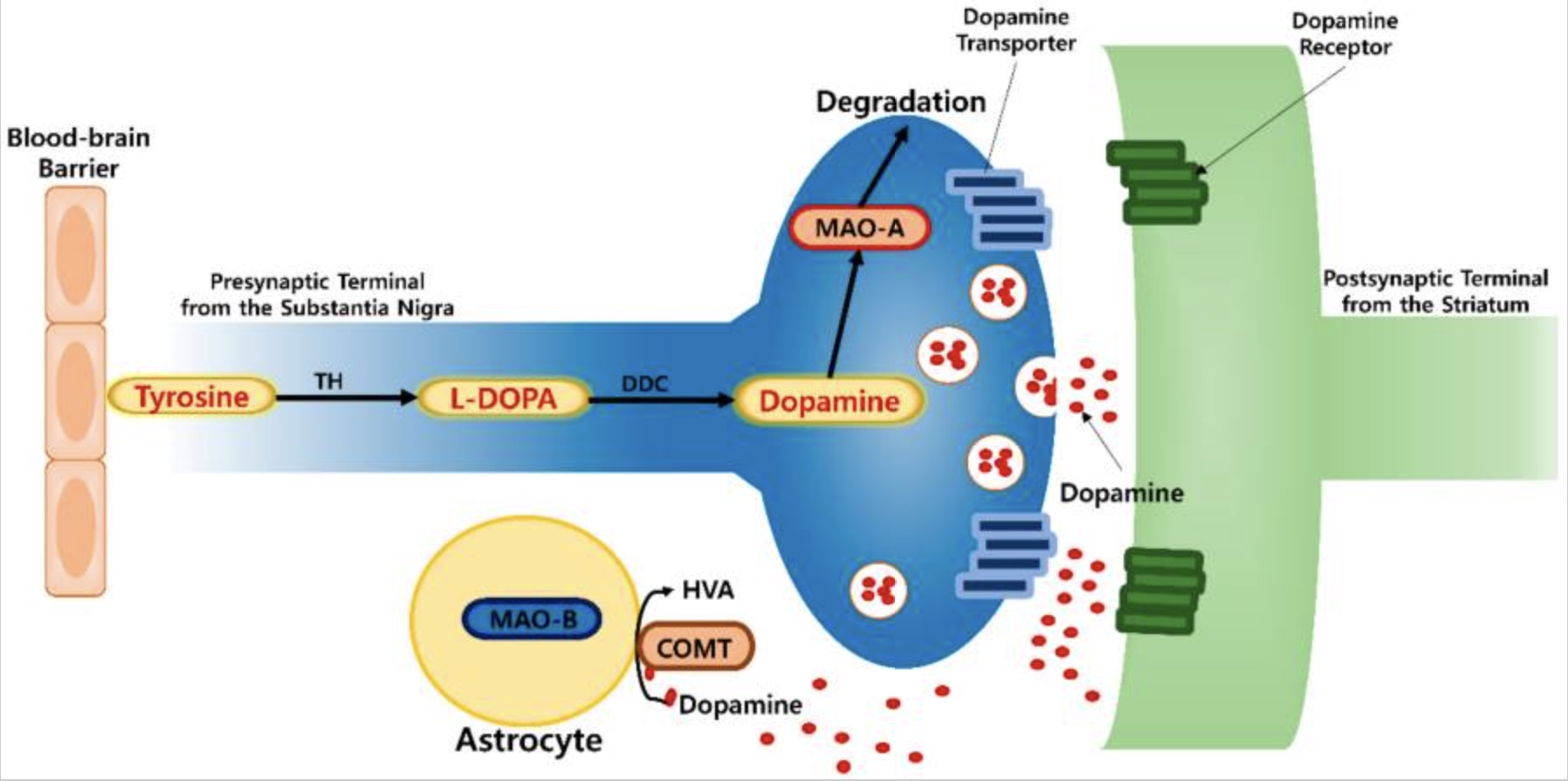
MAO-A is found in the pre-synaptic neurons, controlling how much dopamine, norepinephrine, or serotonin is available to be released.
The MAO-B enzyme is expressed throughout the regions of the brain, primarily in astrocytes but also on the mitochondrial membrane in serotonergic neurons. Astrocytes are a type of brain cell that helps neurons function in a couple of ways, one of which is by clearing excess neurotransmitters.
In the process of metabolizing neurotransmitters, MAO-B uses FAD (derived from riboflavin) and produces hydrogen peroxide.[ref] Excess hydrogen peroxide causes oxidative stress, so MAO-B needs to be maintained at the right levels. More on this in a minute.
Other ways of balancing neurotransmitters:
Before we go any further, I wanted to point out that the MAO enzymes are just one way that serotonin, dopamine, epinephrine, and norepinephrine can be broken down. Other enzymes and pathways also influence the levels of monoamine neurotransmitters.
MAO and Mental Health:
The relationship between MAO levels and mental health is complex.
Imbalanced or rapidly changing neurotransmitter levels can lead to changes in mood and behavior.
Low levels of MAO have been linked to an increased risk for certain mental health conditions such as depression, aggression, and impulsive behavior. It is thought to be related to the role of MAO-A in regulating neurotransmitter levels in the brain. When MAO-A levels are low, neurotransmitters such as serotonin and dopamine may build up in the brain, which can lead to changes in mood and behavior.
On the other hand, studies have shown that individuals with genetically higher levels of MAOA may have a reduced risk for certain mental health conditions. Higher levels of MAOA could decrease neurotransmitter levels to maintain balanced levels of these chemicals in the brain.[ref]
The MAO-A variants can interact with environmental factors to influence mental health. For example, childhood trauma, stress, and exposure to toxins can affect the expression of the MAOA gene, leading to changes in MAO-A levels.[ref]
During childhood and early adolescence, brain structures are rapidly developing. Researchers have found that traumatic events, such as physical or sexual abuse, during childhood can affect adult brain function in some people. The MAO-A genetic variants are linked to an increased risk of aggressive behavior in men exposed to childhood trauma.[ref]
One study found that low MAO-A levels combined with childhood trauma, increased the risk of higher aggression scores in men.[ref]
Stress, Cortisol, and MAO-A:
Stress, whether physical, social, or mental, causes cortisol to be released from the adrenal glands. Cortisol is a hormone that impacts a number of systems in the body including increasing metabolism and reducing immune response, and cortisol can interact with MAO-A.
Acute stress, like a hard workout or a difficult test, causes a swift rise in cortisol levels. Brain imaging studies show that acute stress – and the subsequent cortisol release – reduces whole-brain MAO-A levels. Corticosteroid medications, such as dexamethasone, also significantly decrease MAOA activity.[ref] A sudden suppression of MAO-A could increase dopamine and other neurotransmitter levels, sharpening awareness and quick decision-making, which makes sense in a situation that is suddenly stressful.
However, chronic stress seems to result in the opposite – an increase in MAO-A levels, at least in certain regions of the brain. The increased MAO-A levels in chronic social stress result in decreased serotonin and dopamine release, playing a role in depression from chronic stress.[ref]
MAO-A, MAOIs, and Tyramine:
MAO enzymes, particularly MAO-A, are involved in the breakdown of tyramine, a naturally occurring amine found in certain foods. Tyramine is found in fermented foods, like aged cheese and cured meats, as well as chocolate, non-pasteurized beer, and some wines.
The MAO-A enzyme is one of several enzymes that can break down tyramine.
Related article: Tyramine metabolism
MAO inhibitors (MAOIs) are psychiatric medications that block the MAO-A enzyme, thus increasing serotonin and dopamine levels. They are most commonly prescribed for depression.
When MAO inhibitors (MAOIs) are used as psychiatric medications, they can prevent the breakdown of tyramine, leading to elevated levels in the body. People on MAOIs are cautioned not to eat foods containing high levels of tyramines. When tyramine levels are too high, a hypertensive crisis can occur – dangerously raising blood pressure and causing stroke-like symptoms.[ref]
MAO, Parkinson’s, and Toxins:
While the monoamine oxidase enzymes mainly break down substances produced in the body (e.g. neurotransmitters), they also can interact with the way that a few drugs are broken down and some environmental toxicants. This interaction can play a role in Parkinson’s disease, which is caused by damage to dopaminergic neurons in a specific region of the brain.
One chemical that is metabolized by MAO-B is 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). MPTP itself is non-toxic and can cross the blood-brain barrier. In the brain, MAO-B converts it into a neurotoxin, MPP+, which can cause cell death in dopamine-producing neurons in the substantia nigra. The death of dopamine-producing neurons in the substantia nigra causes Parkinson’s disease.[ref]
MPTP was discovered as a contaminant in illegal drugs that caused immediate and irreversible Parkinson’s symptoms. Within a few years of its discovery, MAO B was identified as the enzyme that turned MPTP into the neurotoxic MPP+. Researchers now use MPP+ extensively to create animal models of Parkinson’s.[ref] You can read more about MPTP and its discovery here.
While you’re not likely to run into MPTP (unless you’re taking synthetic street drugs), it is chemically similar to a commonly used herbicide called paraquat.[ref] Subsequently, a number of studies have shown that higher paraquat exposure increases the risk of Parkinson’s disease.[ref]
Monoamine Oxidase increases ROS:
When monoamine oxidase breaks down a monoamine (neurotransmitters, tyramine, etc.), the reaction produces hydrogen peroxide and possibly ammonia, which are reactive oxygen species (ROS). This production of ROS is normal, and cells produce anti-oxidants to balance out the ROS. In the brain, glutathione is the antioxidant that counteracts the hydrogen peroxide created by MAOs.[ref]
However, when cellular antioxidants are overwhelmed by too much ROS, a state of oxidative stress occurs in the cell. When MAO is upregulated, such as when exposed to lipopolysaccharide (gram-negative bacteria), oxidative stress can occur.
In addition to changing neurotransmitter levels, MAO inhibitor drugs also can decrease inflammation. The research on this is pretty new, but it will be interesting to see whether part of the reason MAO inhibitors work for depression is due, at least in part, to decreasing neuroinflammation, which is a root cause of depression for some people.[ref]
Related article: Inflammation as a cause of depression
Genetics and MAO Levels:
Genetic variants, or SNPs, in the MAOA and MAOB genes can impact the levels of these two enzymes. For MAOA, researchers have discovered common variants (covered below in the genotype report) and also variable number tandem repeats, or repeated sections of the gene, which are unavailable in your genotype data.
Importantly, genetic variants are not the only determinants of mental health. Studies show that the relationship between MAO-A levels and mental health is complex, and can be influenced by various other genetic and environmental factors.
Studies that show no link between a genetic variant and a trait are also important to consider. For example, a study of attention to task found that MAOA variants did not play a role.[ref]
The MAOA and MAOB genes are located on the X chromosome, so men only have one copy of the gene. For women, only one X chromosome is active in a cell, but it can vary in different tissues as to which copy of the X chromosome is silenced.[ref]
Low MAOA: Warrior Gene or Criminal?
MAO-A has been nicknamed the “Warrior” or “Criminal gene” due to early research in mice and in men showing that low MAO-A levels are linked to aggression and impulsiveness. Some studies refer to this as MAOA-L, which stands for low MAO-A.[ref] One study found that the low MAOA (MAOA-L) genotype along with a CDH13 genetic variant was more common in violent criminals.[ref]
Aggression studies in animals, such as those done in rats, clearly point to a role for MAOA in aggression. However, MAOA more strongly affects dopamine levels in rodents than in humans. For example, mice that are not bred to have the MAOA gene are much more aggressive.[ref]
Newer and more in-depth research shows that the MAOA low genotype doesn’t cause aggression in most people. Instead, the research gives us a more nuanced picture of how MAOA variants impact personality. Many studies have tried to tease out the differences between lower or higher MAOA levels. It’s not as simple as “low MAO-A causes aggression”. Instead, research points to the interaction of low MAO-A and childhood neglect or mistreatment in males increasing the relative risk of aggression.[ref]
Really rare mutations that significantly decrease MAOA levels in humans cause a genetic disease called Brunner syndrome, and the symptoms include impulsive and antisocial behavior.[ref] So the effect of low MAOA on impulsivity is real, but it isn’t necessarily caused by the common variants that decrease MAOA.
Benefits of low MAO-A:
With a positive childhood and good parenting, low MAO-A levels are linked to a decreased risk of aggression in both men and women.[ref]
While parental acceptance and involvement benefit all teen boys, a study showed that teen boys with higher MAO-A levels exhibited even higher sensitivity toward parental involvement.[ref]
Interestingly, women with the ‘warrior gene’ version of MAOA are at a lower risk of ADHD.[ref]
COMT and MAOA:
The COMT enzyme also breaks down catecholamines (dopamine, norepinephrine, and epinephrine). It is mainly found in the cytosol of cells, while MAO is found in the mitochondria.[ref]
Recent studies on aggression and mood have looked at the interaction between slow COMT activity along with low MAOA activity.
- In teen boys, having the low versions of both MAOA and COMT was protective against ‘non-suicidal self-injury’. The study was done on males who had been abused as children.[ref]
- An interaction between COMT and MAOB has also been seen in research on the susceptibility to OCD (obsessive-compulsive disorder).[ref] This possible link seems to be specific to MAO B and not MAO-A. Other research points to no interaction between COMT, MOA A, and OCD.[ref]
You can check your COMT variants below and learn more in this article: COMT and Neurotransmitters
MAO Genotype Report:
Members: Log in to see your data below.
Not a member? Join here.
Why is this section is now only for members? Here’s why…
Lifehacks:
If you are a low MAO producer, should you try to increase it? I don’t know that the answer to that question is clear. There may be long-term tradeoffs for higher MAO A or MAO B levels in terms of neurodegenerative diseases.[ref]
If you are on any medication, talk with your doctor before taking any supplements that impact MAO.
The rest of this article is for Genetic Lifehacks members only. Consider joining today to see the rest of this article.
Cofactor for the MAO enzymes:
Related Articles and Topics:
Serotonin: How your genes affect this neurotransmitter
Serotonin is a neurotransmitter that is important in depression, sleep, and many other aspects of health. Learn how your genetic variants in the serotonin receptor genes impact their function.
The Interplay of Genetics and Environment in Parkinson’s Disease
Parkinson’s disease (PD) is not yet fully understood. Researchers think that it is caused by a combo of genetics and environmental factors. Learn more about this disease and the factors that lead to susceptibility.
Resilience: Genetic Variants Involved in Surviving Childhood Trauma
Exposure to childhood trauma, such as exposure to abuse, violence, or repeated stress, can have a long-lasting effect. Genetic differences in the CRHR1 gene are linked to elevated cortisol levels in adults who were exposed to trauma in childhood.
Alpha-1 Antitrypsin Deficiency
A genetic mutation in the SERPINA1 gene causes alpha-1 antitrypsin deficiency. This increases a person’s susceptibility to COPD (chronic obstructive pulmonary disease) and, in some cases, cirrhosis of the liver. Knowing that you carry this mutation can be a great incentive to avoid smoking and to be kind to your liver
References:
Berlowitz, Ilana, et al. “Monoamine Oxidase Inhibition by Plant-Derived β-Carbolines; Implications for the Psychopharmacology of Tobacco and Ayahuasca.” Frontiers in Pharmacology, vol. 13, 2022, p. 886408. PubMed, https://doi.org/10.3389/fphar.2022.886408.
Brunner, H. G., et al. “Abnormal Behavior Associated with a Point Mutation in the Structural Gene for Monoamine Oxidase A.” Science (New York, N.Y.), vol. 262, no. 5133, Oct. 1993, pp. 578–80. PubMed, https://doi.org/10.1126/science.8211186.
Chappell, Kenneth, et al. “The MAOA Rs979605 Genetic Polymorphism Is Differentially Associated with Clinical Improvement Following Antidepressant Treatment between Male and Female Depressed Patients.” International Journal of Molecular Sciences, vol. 24, no. 1, Dec. 2022, p. 497. PubMed Central, https://doi.org/10.3390/ijms24010497.
Chaurasiya, Narayan D., et al. “Natural Products Inhibitors of Monoamine Oxidases—Potential New Drug Leads for Neuroprotection, Neurological Disorders, and Neuroblastoma.” Molecules, vol. 27, no. 13, July 2022, p. 4297. PubMed Central, https://doi.org/10.3390/molecules27134297.
Chen, Ping-Ho, et al. “Genetic and Proteinic Linkage of MAO and COMT with Oral Potentially Malignant Disorders and Cancers of the Oral Cavity and Pharynx.” Cancers, vol. 13, no. 13, June 2021, p. 3268. PubMed Central, https://doi.org/10.3390/cancers13133268.
Chiang, Shang-Lun, et al. “A Haplotype-Specific Linkage Disequilibrium Pattern of Monoamine Oxidase A Gene Associated with Regular Smoking in Women.” The Journal of Gene Medicine, vol. 21, no. 12, Dec. 2019, p. e3142. PubMed, https://doi.org/10.1002/jgm.3142.
Choi, Se Joon, et al. “Changes in Neuronal Dopamine Homeostasis Following 1-Methyl-4-Phenylpyridinium (MPP+) Exposure.” The Journal of Biological Chemistry, vol. 290, no. 11, Mar. 2015, pp. 6799–809. PubMed Central, https://doi.org/10.1074/jbc.M114.631556.
Clukay, Christopher J., et al. “Association of MAOA Genetic Variants and Resilience with Psychosocial Stress: A Longitudinal Study of Syrian Refugees.” PLoS ONE, vol. 14, no. 7, July 2019, p. e0219385. PubMed Central, https://doi.org/10.1371/journal.pone.0219385.
Edmondson, Dale E., et al. “The FAD Binding Sites of Human Monoamine Oxidases A and B.” Neurotoxicology, vol. 25, no. 1–2, Jan. 2004, pp. 63–72. PubMed, https://doi.org/10.1016/S0161-813X(03)00114-1.
Engelbrecht, Idalet, et al. “Evaluation of Selected Natural Compounds as Dual Inhibitors of Catechol-O-Methyltransferase and Monoamine Oxidase.” Central Nervous System Agents in Medicinal Chemistry, vol. 19, no. 2, 2019, pp. 133–45. PubMed, https://doi.org/10.2174/1871524919666190619090852.
Frazzetto, Giovanni, et al. “Early Trauma and Increased Risk for Physical Aggression during Adulthood: The Moderating Role of MAOA Genotype.” PLoS ONE, vol. 2, no. 5, May 2007, p. e486. PubMed Central, https://doi.org/10.1371/journal.pone.0000486.
Gao, Yemiao, et al. “The Effects of Childhood Maltreatment on Non-Suicidal Self-Injury in Male Adolescents: The Moderating Roles of the Monoamine Oxidase A (MAOA) Gene and the Catechol-O-Methyltransferase (COMT) Gene.” International Journal of Environmental Research and Public Health, vol. 18, no. 5, Mar. 2021, p. 2598. PubMed, https://doi.org/10.3390/ijerph18052598.
Goldman, Samuel M., et al. “Genetic Modification of the Association of Paraquat and Parkinson’s Disease.” Movement Disorders : Official Journal of the Movement Disorder Society, vol. 27, no. 13, Nov. 2012, pp. 1652–58. PubMed Central, https://doi.org/10.1002/mds.25216.
Gonzalez, Irene, et al. “MAOB Rs3027452 Modifies Mood Improvement After Tryptophan Supplementation.” International Journal of General Medicine, vol. 14, May 2021, pp. 1751–56. PubMed Central, https://doi.org/10.2147/IJGM.S305443.
Hotamisligil, G. S., and X. O. Breakefield. “Human Monoamine Oxidase A Gene Determines Levels of Enzyme Activity.” American Journal of Human Genetics, vol. 49, no. 2, Aug. 1991, pp. 383–92.
Hwang, In Wook, et al. “Association of Monoamine Oxidase A (MAOA) Gene UVNTR and Rs6323 Polymorphisms with Attention Deficit and Hyperactivity Disorder in Korean Children.” Medicina (Kaunas, Lithuania), vol. 54, no. 3, May 2018, p. 32. PubMed, https://doi.org/10.3390/medicina54030032.
Kolla, Nathan J., and Marco Bortolato. “The Role of Monoamine Oxidase A in the Neurobiology of Aggressive, Antisocial, and Violent Behavior: A Tale of Mice and Men.” Progress in Neurobiology, vol. 194, Nov. 2020, p. 101875. PubMed, https://doi.org/10.1016/j.pneurobio.2020.101875.
Langston, J. William. “The MPTP Story.” Journal of Parkinson’s Disease, vol. 7, no. Suppl 1, pp. S11–19. PubMed Central, https://doi.org/10.3233/JPD-179006. Accessed 7 Feb. 2023.
Lundwall, Rebecca A., and Jeffrey K. Watkins. “Genetic Influence on Slope Variability in a Childhood Reflexive Attention Task.” PLoS ONE, vol. 10, no. 6, June 2015, p. e0130668. PubMed Central, https://doi.org/10.1371/journal.pone.0130668.
Mantas, Ioannis, et al. “TAAR1-Dependent and -Independent Actions of Tyramine in Interaction With Glutamate Underlie Central Effects of Monoamine Oxidase Inhibition.” Biological Psychiatry, vol. 90, no. 1, July 2021, pp. 16–27. PubMed, https://doi.org/10.1016/j.biopsych.2020.12.008.
Martínez, Róger Marcelo, et al. “Interaction Effects of the 5-HTT and MAOA-UVNTR Gene Variants on Pre-Attentive EEG Activity in Response to Threatening Voices.” Communications Biology, vol. 5, Apr. 2022, p. 340. PubMed Central, https://doi.org/10.1038/s42003-022-03297-w.
McGregor, N. W., et al. “Modification of the Association between Early Adversity and Obsessive-Compulsive Disorder by Polymorphisms in the MAOA, MAOB and COMT Genes.” Psychiatry Research, vol. 246, Dec. 2016, pp. 527–32. PubMed, https://doi.org/10.1016/j.psychres.2016.10.044.
Mentis, Alexios-Fotios A., et al. “From Warrior Genes to Translational Solutions: Novel Insights into Monoamine Oxidases (MAOs) and Aggression.” Translational Psychiatry, vol. 11, Feb. 2021, p. 130. PubMed Central, https://doi.org/10.1038/s41398-021-01257-2.
Meulendyke, Kelly A., et al. “Elevated Brain Monoamine Oxidase Activity in SIV- and HIV-Associated Neurological Disease.” The Journal of Infectious Diseases, vol. 210, no. 6, Sept. 2014, pp. 904–12. PubMed Central, https://doi.org/10.1093/infdis/jiu194.
Raghu, Ganesh, et al. “The Multifaceted Therapeutic Role of N-Acetylcysteine (NAC) in Disorders Characterized by Oxidative Stress.” Current Neuropharmacology, vol. 19, no. 8, Aug. 2021, pp. 1202–24. PubMed Central, https://doi.org/10.2174/1570159X19666201230144109.
Rendić, Slobodan P., et al. “Roles of Selected Non-P450 Human Oxidoreductase Enzymes in Protective and Toxic Effects of Chemicals: Review and Compilation of Reactions.” Archives of Toxicology, vol. 96, no. 8, 2022, pp. 2145–246. PubMed Central, https://doi.org/10.1007/s00204-022-03304-3.
Sampaio, Aline Santos, et al. “COMT and MAO-A Polymorphisms and Obsessive-Compulsive Disorder: A Family-Based Association Study.” PloS One, vol. 10, no. 3, 2015, p. e0119592. PubMed, https://doi.org/10.1371/journal.pone.0119592.
Santos, Beatriz Werneck Lopes, et al. “Biodiversity of β-Carboline Profile of Banisteriopsis Caapi and Ayahuasca, a Plant and a Brew with Neuropharmacological Potential.” Plants, vol. 9, no. 7, July 2020, p. 870. PubMed Central, https://doi.org/10.3390/plants9070870.
Shih, J. C., et al. “Ginkgo Biloba Abolishes Aggression in Mice Lacking MAO A.” Antioxidants & Redox Signaling, vol. 2, no. 3, 2000, pp. 467–71. PubMed, https://doi.org/10.1089/15230860050192242.
Sturza, Adrian, et al. “Monoamine Oxidase-Related Vascular Oxidative Stress in Diseases Associated with Inflammatory Burden.” Oxidative Medicine and Cellular Longevity, vol. 2019, Apr. 2019, p. 8954201. PubMed Central, https://doi.org/10.1155/2019/8954201.
Sun, Yan, et al. “MAOA Rs1137070 and Heroin Addiction Interactively Alter Gray Matter Volume of the Salience Network.” Scientific Reports, vol. 7, Mar. 2017, p. 45321. PubMed, https://doi.org/10.1038/srep45321.
Tadić, André, et al. “A Monoamine Oxidase B Gene Variant and Short-Term Antidepressant Treatment Response.” Progress in Neuro-Psychopharmacology & Biological Psychiatry, vol. 31, no. 7, Oct. 2007, pp. 1370–77. PubMed, https://doi.org/10.1016/j.pnpbp.2007.05.015.
Tiihonen, J., et al. “Genetic Background of Extreme Violent Behavior.” Molecular Psychiatry, vol. 20, no. 6, June 2015, pp. 786–92. PubMed Central, https://doi.org/10.1038/mp.2014.130.
Truman, Penelope, et al. “Monoamine Oxidase Inhibitory Activity of Flavoured E-Cigarette Liquids.” Neurotoxicology, vol. 75, Dec. 2019, pp. 123–28. PubMed, https://doi.org/10.1016/j.neuro.2019.09.010.
Tu, Hung-Pin, et al. “Monoamine Oxidase A Gene Polymorphisms and Enzyme Activity Associated with Risk of Gout in Taiwan Aborigines.” Human Genetics, vol. 127, no. 2, Feb. 2010, pp. 223–29. PubMed, https://doi.org/10.1007/s00439-009-0765-z.
Tucker, Donovan, et al. “From Mitochondrial Function to Neuroprotection – An Emerging Role for Methylene Blue.” Molecular Neurobiology, vol. 55, no. 6, June 2018, pp. 5137–53. PubMed Central, https://doi.org/10.1007/s12035-017-0712-2.
Xu, Xiaohui, et al. “Association Study between the Monoamine Oxidase A Gene and Attention Deficit Hyperactivity Disorder in Taiwanese Samples.” BMC Psychiatry, vol. 7, Feb. 2007, p. 10. PubMed, https://doi.org/10.1186/1471-244X-7-10.
Yen, Ju-Yu, et al. “Roles of Hostility and Depression in the Association between the MAOA Gene Polymorphism and Internet Gaming Disorder.” International Journal of Environmental Research and Public Health, vol. 18, no. 13, June 2021, p. 6910. PubMed Central, https://doi.org/10.3390/ijerph18136910.
Zhang, Wenxin, et al. “Monoamine Oxidase A (MAOA) and Catechol-O-Methyltransferase (COMT) Gene Polymorphisms Interact with Maternal Parenting in Association with Adolescent Reactive Aggression but Not Proactive Aggression: Evidence of Differential Susceptibility.” Journal of Youth and Adolescence, vol. 45, no. 4, Apr. 2016, pp. 812–29. PubMed, https://doi.org/10.1007/s10964-016-0442-1.
Zhao, Bao, et al. “Parenting Practices and Adolescent Effortful Control: MAOA T941G Gene Polymorphism as a Moderator.” Frontiers in Psychology, vol. 11, 2020, p. 60. PubMed, https://doi.org/10.3389/fpsyg.2020.00060.