Key takeaways:
~ Chronically elevated inflammation causes depression and anxiety, for some people…
~ Genetics may help point to whether this is an underlying cause for you.
~ Finding your underlying physiological cause of depression or anxiety may lead you to the solutions that are right for you.
This article explains why inflammation causes depression and how your genetic variants in inflammatory genes may play a role in depression or anxiety. Members will see their genotype report below, plus additional solutions in the Lifehacks section. Join today.
Depression and Anxiety: Inflammation as a root cause
Research over the past two decades clearly shows a causal link between increased inflammatory markers and depression.
This means that inflammation – elevated levels of inflammatory cytokines – can CAUSE mood disorders.
Theories about depression and anxiety have changed over the years. Researchers now think that depression, anxiety, and mood issues can have multiple causes. For a good percentage of people with mood disorders, though, addressing inflammation as an underlying factor helps to resolve depression or anxiety.[ref][ref]
Inflammation-driven depression is characterized by:[ref]
- sleep problems
- tiredness, lack of energy
- changes in appetite
- anhedonia (meh, feeling like nothing matters)
Cause or effect?
Research shows increased inflammatory cytokine levels in some depression patients.
This is interesting, but it doesn’t tell us whether depression causes inflammation – or – if inflammation causes depression. Or if both simply exist together.[ref]
So how can we narrow it down? Genetic studies and animal research.
Genetics:
One way to determine if inflammation is a root cause of mood disorders is to look at the genetic variants that increase susceptibility to depression or anxiety. Sure enough, research shows a bunch of genetic risk factors for mood disorders are in inflammation-related genes.[ref] Deleting those inflammation genes causes animals to be resistant to stress-induced depression.[ref]
Other ways of determining causality:
Animal models of depression let researchers experiment to determine causality. Increasing inflammatory cytokines clearly causes depression and anxiety symptoms in animals, and decreasing the inflammation takes away the symptoms.[ref] Deleting certain inflammation genes causes animals to be resistant to stress-induced depression.[ref] Additionally, human studies show drugs that decrease inflammation significantly improve depression – in some people.[ref]
Disclaimer: This article is a presentation of the research on depression, anxiety, and inflammation. It is not to be taken as medical advice. If you are under the care of a physician for a mood disorder, please talk with your provider for medical advice.
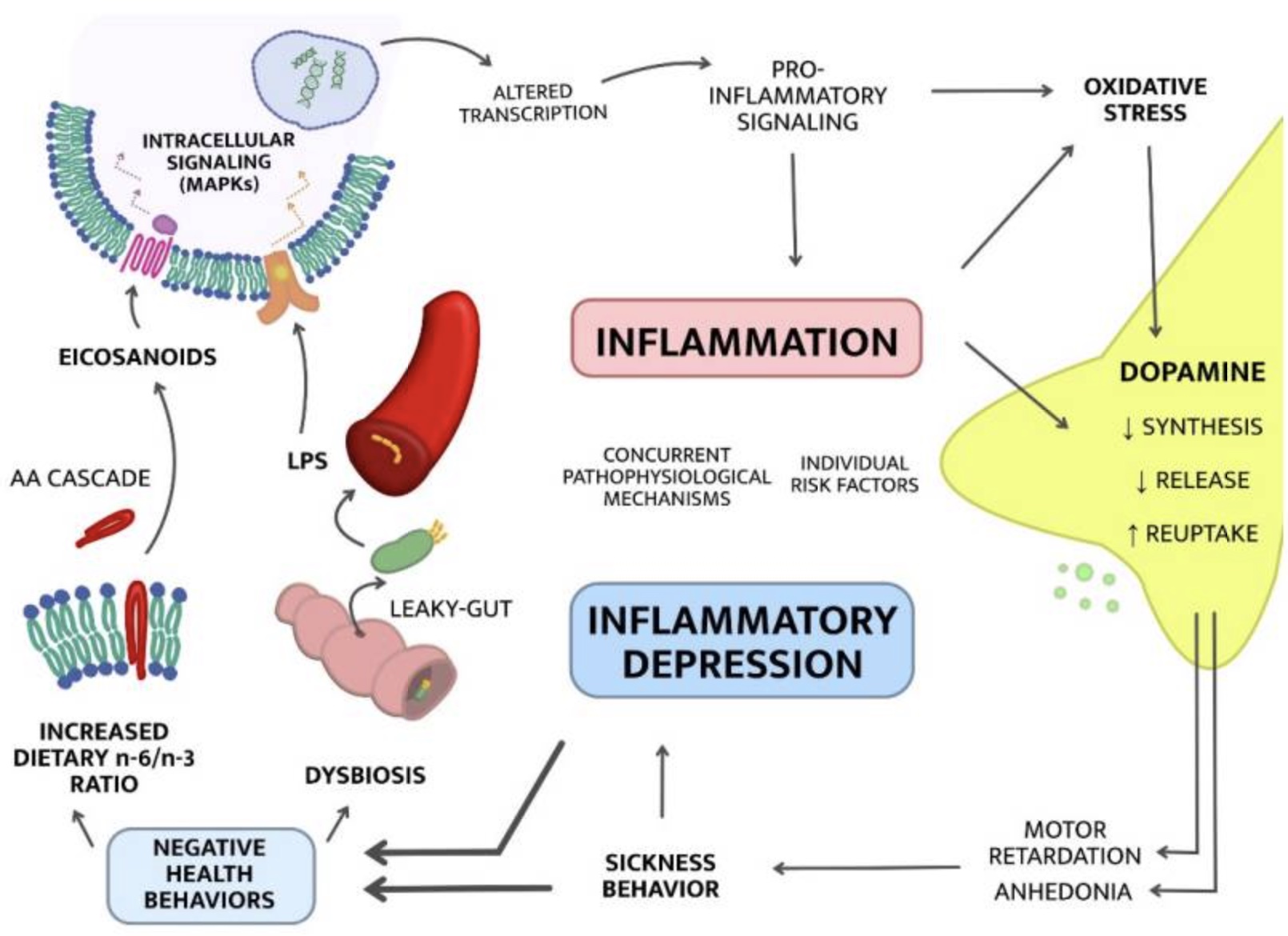
Inflammatory cytokines cause behavior changes:
Behavior changes when you get sick. You’re tired, irritable, and don’t want to interact with people…
If you have kids, you learn to spot these signs they fighting off ‘a bug’ very quickly.
Even in pets, you will see that they withdraw, sleep, and avoid others when they are sick. Appetite changes, and they often are hyper-vigilant as well.
Researchers call this ‘sickness behavior‘, and it’s apparent across all species of animals. It’s instinctive.
Sickness behavior makes complete sense evolutionarily when you think about it. The best way not to infect your family (or herd, pack, tribe, flock, group) is to go and curl up in a corner and avoid everyone.
We have always known that illness causes behavior changes, but the connection between psychiatric illness and inflammatory response was made crystal clear in the 1980s.
Interferon-alpha, an inflammatory cytokine that fights viruses, was first used in the 80s as a treatment for chronic hepatitis. Some patients reacted immediately to the interferon-alpha with psychiatric side effects, including depression, emotional instability, paranoia, agitation, and suicidal potential. These side effects occurred in patients on the highest doses of interferon-alpha, and reducing the dose took away the psychiatric problems.[ref][ref][ref]
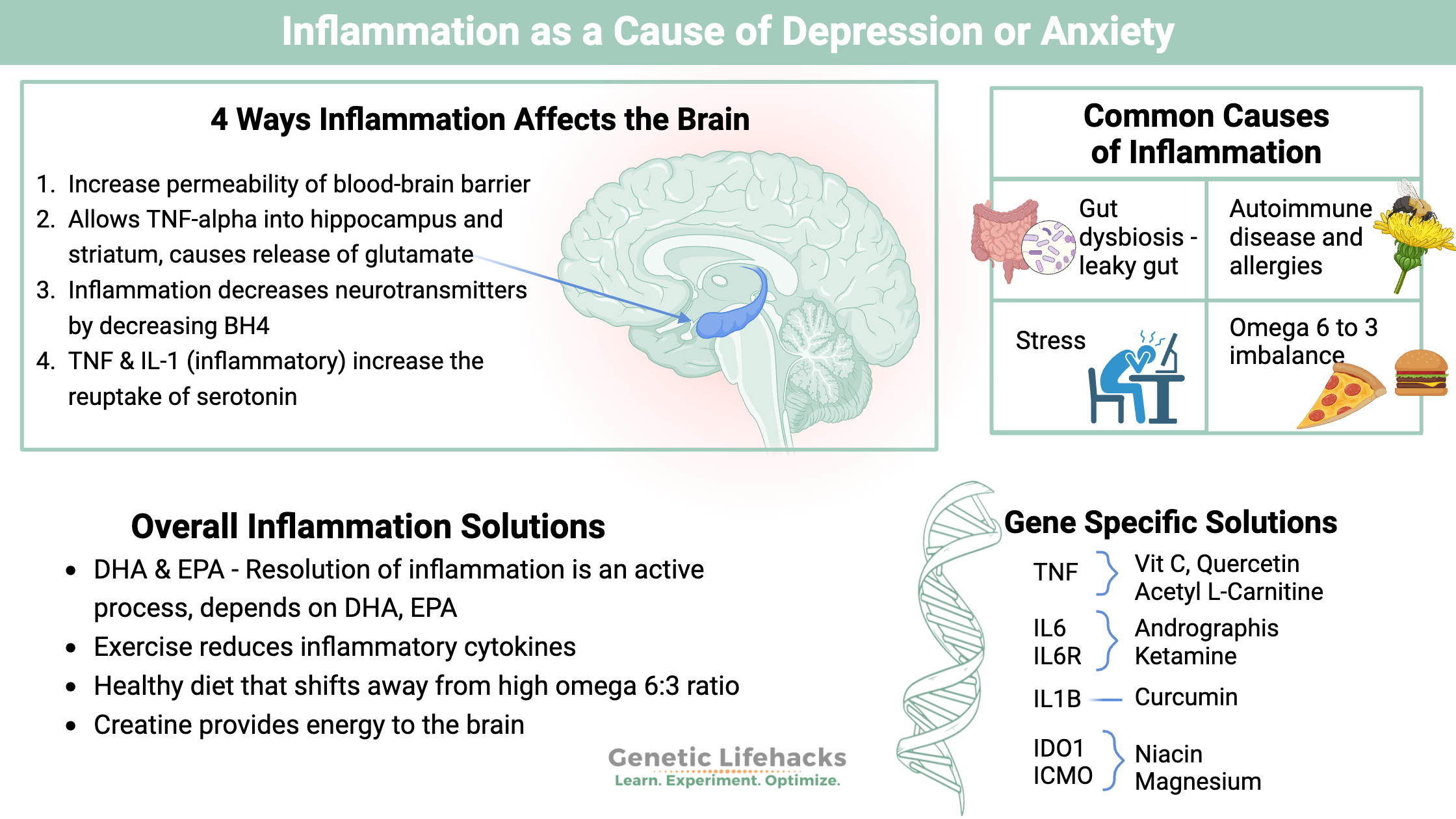
5 common causes of increased inflammation:
While this is a huge topic, when it comes to inflammation and depression, there are a couple of key lifestyle factors that may come into play and explain why depression and anxiety are so prevalent today. The following four factors are common causes of elevated cytokines (inflammatory molecules).
#1 Gut dysbiosis and increased permeability:
Your intestinal barrier keeps the microbes in your gut separate from the rest of your body. Bacteria can make you sick, but the microbes in your gut microbiome are also essential for good health. The difference between good gut microbes and getting sick is the barrier that keeps the microbes in the right place.
Lining your intestines is a mucosal barrier that keeps the gut microbes away from the epithelial cell wall. Those epithelial cells also keep out microbes by being tightly joined together. Reducing the mucosal barrier and/or loosening the tight junctions in your intestinal barrier can increase the number of microbes that your body needs to fight off.
One study on patients with major depressive disorder showed that patients have higher than average activity of the inflammatory markers for dealing with bacteria from the gut.[ref]
What can cause gut dysbiosis and increased intestinal permeability? A diet that includes a lot of junk food. (read about emulsifiers, food additives, and inflammation)
#2 Autoimmune diseases and allergies:
Many autoimmune diseases, including lupus, multiple sclerosis, psoriasis, and rheumatoid arthritis, have links to an increase in inflammation. People with these autoimmune issues are also at an increased risk of depression. Allergies and asthma also have links to an increased risk of depression. For example, a meta-analysis calculated that the prevalence of depression in people with rheumatoid arthritis is ~39%.[ref][ref][ref][ref][ref][ref]
#3 Stress:
The body undergoes a series of physical changes when stressed. Major life events, such as losing a job, the death of a loved one, moving, or divorce, cause stress in the body. While it may be unsurprising that these events have links to depression, the physical cause of behavior changes may be due to an increase in inflammatory cytokines. For our ancestors, the stress response was often due to physical events such as an attack by a wild animal. When the stress response initiates, pro-inflammatory cytokines upregulate to enhance wound healing and improve physical recovery. So, when a boss yells at you, or you become the target of negative tweets, the body’s stress response still causes an increase in pro-inflammatory cytokines, even if no physical wound exists.[ref]
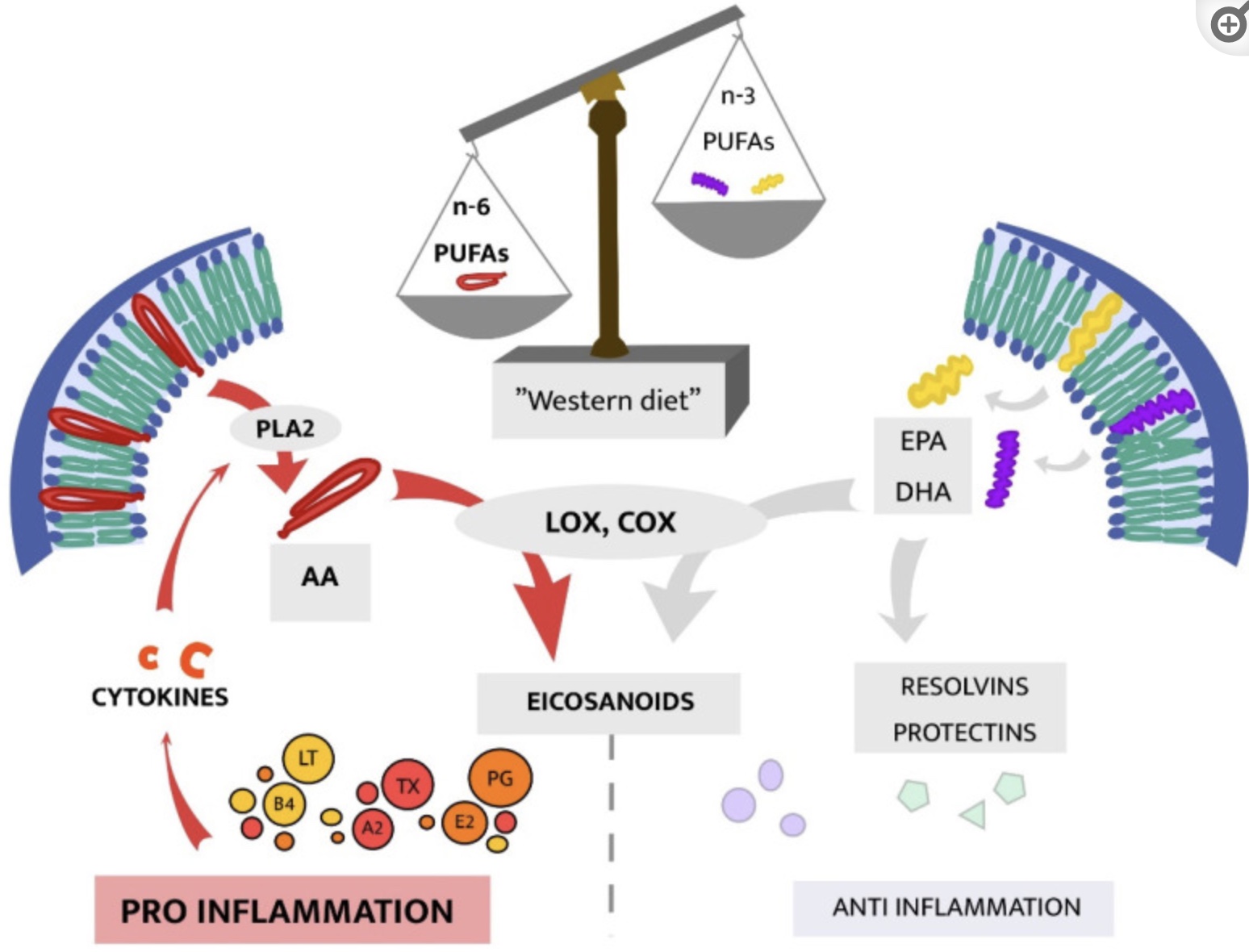
#4 Omega 6: Omega 3 ratio:
Another source of increased overall inflammation is consuming a higher ratio of omega-6 to omega-3 fats. Omega 6 fats are found in oils such as soybean, corn, vegetable oil, sunflower, and other seed oils. Our modern diet has had a huge shift in the amount of omega-6 fats we consume. It turns out that while omega-6 fats may be good in small amounts, this dietary shift to a lot of omega-6 oils causes increases in overall inflammation.[ref]
Recently, scientists have discovered that the resolution of inflammation is an active process that involves specific lipid mediators. The omega-3 fatty acids DHA and EPA are the biological starting points for the cells to create lipid mediators that resolve inflammation.[ref]
Related article: Resolution of inflammation and specialized pro-resolving mediators
#5 Obesity:
Higher overall inflammation goes hand-in-hand with weight gain. Adipose tissue (fat) gives off inflammatory cytokines, increasing the overall burden of inflammation. People who are overweight and have depression also have higher inflammatory markers, on average, than normal-weight people with depression.[ref]
One study reports that severely obese people (BMI ≥ 40 kg/m2), are at a much higher risk of depression than non-obese people. It concludes: “The association between adiposity, systemic inflammation, and neuropsychiatric alterations supports the contribution of adiposity-related inflammatory processes to neuropsychiatric comorbidities in obesity.”[ref]
Why does inflammation affect the brain and mood?
Okay, so there are a number of ways that the body can have elevated inflammatory cytokines. But what does this have to do with the brain and mood?
There are a couple of ways that inflammation directly affects the brain:
Increased peripheral inflammatory cytokines directly increase the permeability of the blood-brain barrier. This allows the inflammatory cytokines to enter the brain, triggering functional changes in certain brain regions.
- Higher levels of TNF-alpha in the hippocampus and striatum have links to anxiety and depression in animal studies. TNF-alpha causes the release of glutamate in the hippocampus, causing depression behavior in animals.
- IL-1B decreases neurogenesis via the kynurenine pathway.[ref]
- Inflammation decreases the synthesis of neurotransmitters through the disruption of BH4.
- TNF-alpha and IL-1 increase the reuptake of serotonin, leaving less serotonin available.[ref]
Generally, increased inflammation shifts the body from converting tryptophan into serotonin and instead produces kynurenine, which has a neurotoxic metabolite called quinolinic acid.[ref] (We’ll come back to this in more detail below…)
Inflammatory cytokines can disrupt the production of BH4 (tetrahydrobiopterin), which is needed for the production of dopamine and serotonin.[ref]
Which inflammatory cytokines are linked to depression or anxiety?
Research shows that some people with depression have higher levels of TNFα, IL-6, IL-13, IL-18, IL-12, and IL-1RA.[ref] The “IL” stands for interleukin, which is a whole family of inflammatory molecules.
Studies also show that TNF-alpha, IL-6, and C-reactive protein have links to anxiety disorders in some people.[ref]
Studies on PTSD, which is grouped in with anxiety disorders, show that trauma elevates IL-1B, Il-6, TNF-alpha, and C-reactive protein. Generalized anxiety disorder (GAD) has links to increased levels of TNF-alpha and CRP.[ref]
In fact, to create an animal model of anxiety or depression, researchers subject animals to mild, unpredictable stress (tilting the cage, intermittent light, noise).
Brain imaging studies point to higher levels of CRP (C-reactive protein), which is a general marker of inflammation and is predictive of psychosis. Additionally, increasing IL-10, which is anti-inflammatory, is linked with greater symptom improvement in depression.[ref]
Scientists have also narrowed down that stress-induced depression is more likely to have pro-inflammatory cytokines as a cause.[ref]
Diving deeper into TNF-alpha
TNF-alpha (tumor necrosis factor alpha) is one of the body’s primary inflammatory cytokines. Its production occurs by activated immune cells, such as macrophages, mast cells, B cells, and lymphocytes. It can also be produced in other cells, such as smooth muscle cells, in response to an injury. Adipose tissue (fat tissue) also secretes TNF-alpha.[ref][ref]
When TNF-alpha elevates in depressed people, blocking TNF-alpha can ameliorate depressive symptoms. A 2008 genome-wide association study found that a TNF genetic variant increases the risk of depression.[ref]
People with rheumatoid arthritis are often given drugs to decrease TNF-alpha. The anti-TNF drugs decrease the frequency of anxiety disorder and depression in people with RA.[ref]
Researchers think that inflammation, specifically high TNF-alpha levels, impacts the HPA axis and elevates cortisol as well. HPA axis dysfunction is strongly linked to depression.
Related article: HPA axis dysfunction: genes and environment
Additionally, higher TNF-alpha levels can cause increased uptake of serotonin into cells, causing depressive symptoms.[ref]
If you have ever been told ‘it’s all in your head”, physiologically that could be true for some people with TNF-alpha variants. A brain-imaging study found that a TNF-alpha genetic variant associated with higher TNF levels was linked to anatomical brain changes in people with major depressive disorder.[ref]
Elevated Interleukin-6
Encoded by the IL6 gene, interleukin-6 (IL-6) is another inflammatory cytokine shown to be consistently elevated in depression.
Animal studies show that inducing an elevated level of IL-6 causes depressive behavior. This can be done using stressful situations or by injecting lipopolysaccharide, an endotoxin from bacteria.[ref] Animals bred to not produce IL-6 are resistant to despair behavior usually induced by stress.[ref]
Genetic studies in humans also link higher levels of IL-6, stressful life events, and an increased risk of depression.[ref]
Interleukin-6 is one of the major cytokines released by immune cells in response to a pathogen or toxin. Chronic or acute stress can also cause the release of IL-6. In the brain, increased levels of IL-6 cause decreased neurotransmitters, including serotonin. Additionally, IL-6 causes a decrease in brain-derived neurotrophic factor (BDNF) and impacts the HPA axis. All of these pathways are implicated in mood disorders.[ref]
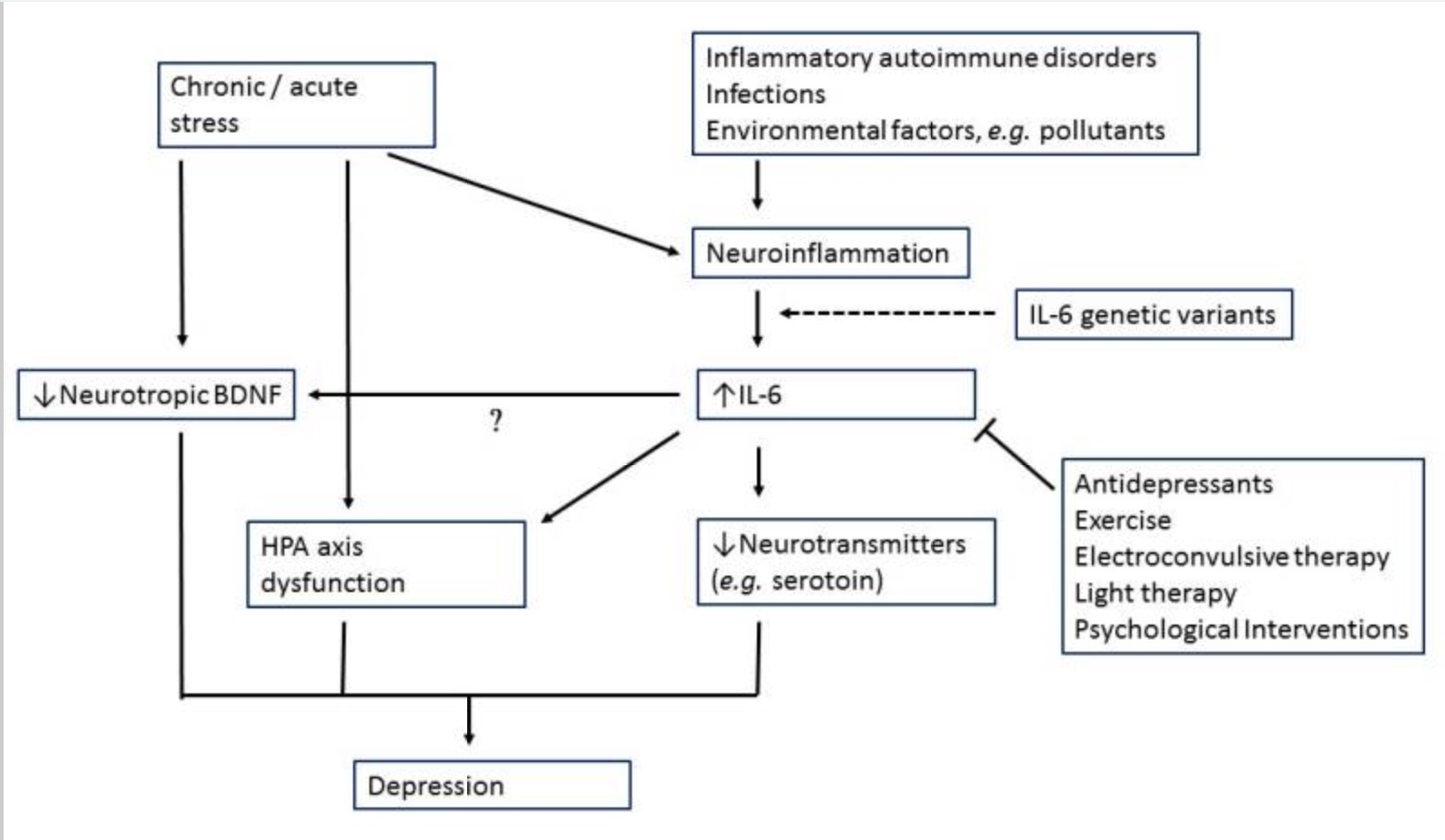
IL-6 also impacts the amygdala’s response to socially threatening situations linking the increased immune response to greater social disconnect, fatigue, and cognitive disturbance.[ref]
Kynurenine pathway:
Tryptophan is an essential amino acid found in many foods that contain protein.
In the body, tryptophan can go down two different pathways:
- tryptophan can be used to make serotonin and melatonin
- tryptophan can convert into kynurenine and then into several other metabolites, including quinolinic acid.
Kynurenine can form quinolinic acid, which is a neurotoxin. This is part of the pathway, though, that eventually produces niacin (vitamin B3).
Tryptophan metabolism needs to balance out. The body and brain need serotonin as an important neurotransmitter. Melatonin, produced from serotonin, is an integral part of the circadian system as well as a vital anti-inflammatory molecule.
Increased inflammation causes a shift from tryptophan being used for serotonin to being used for the kynurenine pathway. This causes a double whammy – lower serotonin and higher quinolinic acid. Quinolinic acid acts on NMDA receptors, and too much can kill off brain cells. Studies show that depression scores correlate to higher quinolinic acid in the blood, and postmortem studies show more cells in the brain producing quinolinic acid in suicide victims.[ref]
Hepatitis C patients treated with interferon-alpha have depression and higher kynurenine metabolites.[ref]
Another reason tryptophan metabolism could tilt more towards kynurenine is when the body is lacking in niacin. Providing enough niacin through the diet is important here.
Genetic variants in the IDO1 gene also can skew the body towards producing kynurenine instead of serotonin. (Read more about tryptophan, kynurenine, serotonin, and genetics.)
Allergies, COVID-19, and depression and anxiety:
A study of the psychological impact of COVID-19 revealed that people with allergies, which chronically raise inflammatory cytokine levels, were more likely to have PTSD and depression after COVID-19.[ref]
Another study found that people with systemically elevated inflammation after COVID-19 illness were at an increased risk of depression, anxiety, or PTSD.[ref]
While there is a lot of swirl and science about long-haul COVID symptoms, previous research on other viruses that remain active or reactivate (e.g., Epstein-Barr virus, cytomegalovirus) also has links between infection, inflammatory cytokines, and depression.[ref]
Inflammation, Depression, and Anxiety: Genotype Report
Not a member? Join here.
Why is this section is now only for members? Here’s why…
Lifehacks:
Talk with your health care provider about any supplements or changes – especially if you are on medications.
The lifehacks below deal with ways to suppress inflammatory cytokines, but the obvious solution is to take away the source of the inflammation. Thus, these lifehacks may help in the immediate timeframe, but for a long-term solution, you need to figure out the source of your inflammation (e.g., obesity, stress, diet, gut dysbiosis).
You may find that multiple solutions stacked together work best. For example, curcumin + exercise + an anti-inflammatory diet may be more effective than just popping a curcumin supplement.
OTC anti-inflammatory options:
Aspirin is a COX-1 and COX-2 inhibitor, which reduces TNF-alpha, IL-6, and CRP – somewhat. Human studies on aspirin for depression have been small and inconclusive, but research does show that aspirin, along with an SSRI, shortened the time it took for the antidepressant to work.[ref]
Naproxen and ibuprofen reduced depression scores in people who also had osteoarthritis.[ref] Studies on NSAIDs in people without an inflammatory condition show no results for depression.
Antibiotics:
Minocycline, a broad-spectrum antibiotic, easily crosses the blood-brain barrier. A recent clinical trial shows that it is effective as an adjunctive treatment along with immunomodulatory medications for treatment-resistant major depressive disorder.[ref]
Minocycline has also been shown in animal studies to work on anxiety.[ref] A 2021 study showed that adding minocycline to antidepressant treatment in people with elevated C-reactive protein significantly reduced depression levels. The results showed that study participants with high IL-6 levels were much more likely to respond to minocycline.[ref]
Related Articles and Topics:
Lithium Orotate and Vitamin B12: Benefits for Mood and Cognitive Support