Key takeaways:
~ The MTHFR C677T and A1298C variants get a lot of press – from Facebook groups to whole websites that talk about them.
~ Additional variants in the MTHFR gene can also affect the function of the enzyme, increasing or decreasing your risk of negative effects from a lack of folate.
~ This article gives you the bigger picture, including MTHFR variants with positive or protective outcomes.
Going beyond MTHFR 677 and 1298
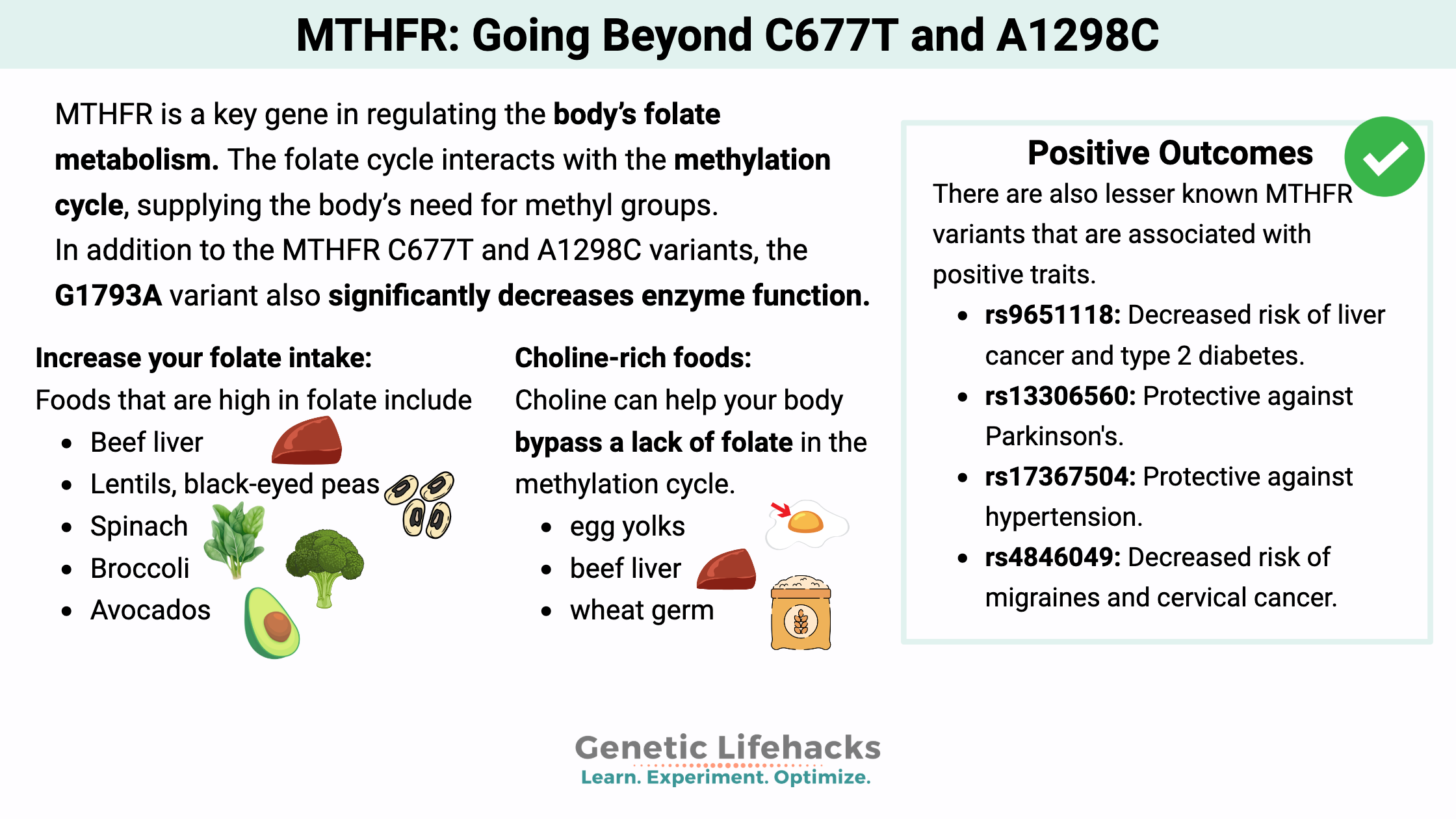
MTHFR is a key gene in regulating the body’s folate metabolism. The folate cycle interacts with the methylation cycle, supplying the body’s need for methyl groups.
I’m assuming that you already know a little about the methylation cycle and the MTHFR C677 and A1298C variants. If you don’t know your status on those two, check out this page: MTHFR: How to check your data for C677T and A1298C, and read through the background information on the methylation cycle.
In a nutshell, the methylation cycle is a cellular cycle responsible for creating methyl groups (CH3). These methyl groups are used by the body in tons of different reactions as well as to modify gene expression. Thus, alterations to the availability of methyl groups can have a wide range of impacts.
When reading about MTHFR, most articles only cover the C677T and A1298C variants. But those two variants do not give the whole picture for the MTHFR gene. Other variants also impact the way the MTHFR enzyme functions – both positively and negatively.
MTHFR G1793A:
While not quite as well researched as the C677T variant, the G1793A variant also has more than a hundred studies on it, showing that it also decreases the function of the MTHFR enzyme. However, the results in studies seem to vary by ancestry. In Caucasian populations, multiple studies point to a decrease in folate conversion and an increase in the risk of cardiovascular disease. In Chinese populations, this variant is protective against diabetes.[ref] The difference may be that dietary betaine (choline) levels differ in traditional Chinese diets compared to Western diets.
Additive effect: A study looking at risk factors for coronary artery disease (CAD) showed that the MTHFR C677T variant increased the relative risk of CAD significantly. The study also found that the G1793A variant (A allele) added to the increased risk of CAD.[ref]
MTHFR rs4846049:
The rs4846049 variant in MTHFR changes the way that a microRNA binds to the mRNA.
Genes are transcribed into mRNA and then translated into their protein – in this case the MTHFR enzyme. Cells have ways of preventing the mRNA from being translated into the protein in order to regulate the amount of the MTHFR enzyme available at a given moment.
MicroRNAs are short strands of RNA that can bind to an mRNA strand and prevent it from being translated into a protein/enzyme.
The rs4846049 variant causes a small change in the MTHFR mRNA that impairs the binding of miR-149 (microRNA 149). This means that the variant causes a little more MTHFR enzyme to be available under certain cellular conditions, which then results in a decreased risk of heart disease.[ref]
Related article: microRNAs
Putting it into perspective:
The variants in MTHFR with both positive and negative impacts add together with your diet and lifestyle. Many of the negative impacts of MTHFR variants are mitigated through a diet rich in folate or choline.[ref]
MTHFR Genotype Report:
Access this content:
An active subscription is required to access this content.
Lifehacks:
If you have variants that decrease your methylation cycle function, you may want to bring your levels up to the normal range.
Increase your folate intake:
Foods that are high in folate include:[ref]
- Beef liver
- Lentils, black-eyed peas
- Spinach
- Broccoli
- Avocados
Read more: Folate-rich foods and recipe ideas
If you don’t eat enough folate-rich foods regularly, methylfolate supplements are also available. The RDA for folate is 400 mcg/day for adults (600 mcg/day for pregnancy). Tracking your folate intake using an app, such as Cronometer, for a week or two should give you a good idea of how much folate you regularly eat.
Choline-rich foods:
Choline can help your body bypass a lack of folate in the methylation cycle.[ref][ref]
Good sources of choline include egg yolks, beef liver, and wheat germ. A metabolite of choline, betaine, is actually what works via the methylation cycle; therefore, food sources of betaine (beets, quinoa, and spinach) are also helpful here. Supplemental betaine (also called TMG) is also available.
Read more: Choline-rich foods and recipes
Supplements and Lifehacks:
Access this content:
An active subscription is required to access this content.
Related Articles and Topics:
References:
Abhinand, P. A., et al. “Insights on the Structural Perturbations in Human MTHFR Ala222Val Mutant by Protein Modeling and Molecular Dynamics.” Journal of Biomolecular Structure & Dynamics, vol. 34, no. 4, 2016, pp. 892–905. PubMed, https://doi.org/10.1080/07391102.2015.1057866.
Aneji, Chiamaka N., et al. “Deep Sequencing Study of the MTHFR Gene to Identify Variants Associated with Myelomeningocele.” Birth Defects Research. Part A, Clinical and Molecular Teratology, vol. 94, no. 2, Feb. 2012, pp. 84–90. PubMed, https://doi.org/10.1002/bdra.22884.
Beydoun, May A., et al. “One-Carbon Metabolism Gene Polymorphisms Are Associated with Cognitive Trajectory among African-American Adults.” Neurobiology of Aging, vol. 84, Dec. 2019, p. 238.e5-238.e18. PubMed, https://doi.org/10.1016/j.neurobiolaging.2019.05.013.
Cai, Can, et al. “Association of MTHFR, SLC19A1 Genetic Polymorphism, Serum Folate, Vitamin B12 and Hcy Status with Cognitive Functions in Chinese Adults.” Nutrients, vol. 8, no. 10, Oct. 2016, p. E665. PubMed, https://doi.org/10.3390/nu8100665.
de Aquino, Sibele Nascimento, et al. “MTHFR Rs2274976 Polymorphism Is a Risk Marker for Nonsyndromic Cleft Lip with or without Cleft Palate in the Brazilian Population.” Birth Defects Research. Part A, Clinical and Molecular Teratology, vol. 100, no. 1, Jan. 2014, pp. 30–35. PubMed, https://doi.org/10.1002/bdra.23199.
Ganz, Ariel B., et al. “Genetic Impairments in Folate Enzymes Increase Dependence on Dietary Choline for Phosphatidylcholine Production at the Expense of Betaine Synthesis.” The FASEB Journal, vol. 30, no. 10, Oct. 2016, pp. 3321–33. PubMed Central, https://doi.org/10.1096/fj.201500138RR.
García, Silvia, et al. “Analysis of the Rs13306560 Functional Variant in the Promoter Region of the MTHFR Gene in Sporadic Parkinson´s Disease.” Neuro Endocrinology Letters, vol. 38, no. 4, Aug. 2017, pp. 257–60.
García-Minguillán, Carlos J., et al. “Riboflavin Status Modifies the Effects of Methylenetetrahydrofolate Reductase (MTHFR) and Methionine Synthase Reductase (MTRR) Polymorphisms on Homocysteine.” Genes & Nutrition, vol. 9, no. 6, Nov. 2014, p. 435. PubMed, https://doi.org/10.1007/s12263-014-0435-1.
Hustad, Steinar, et al. Riboflavin and Methylenetetrahydrofolate Reductase. Landes Bioscience, 2013. www.ncbi.nlm.nih.gov, https://www.ncbi.nlm.nih.gov/books/NBK6145/.
Jadavji, Nafisa M., et al. “B-Vitamin and Choline Supplementation Increases Neuroplasticity and Recovery after Stroke.” Neurobiology of Disease, vol. 103, July 2017, pp. 89–100. PubMed, https://doi.org/10.1016/j.nbd.2017.04.001.
Office of Dietary Supplements – Folate. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/. Accessed 28 Dec. 2021.
Salehi, Mohaddeseh, et al. “The Rs4846049 Polymorphism in the 3’UTR Region of the MTHFR Gene Increases the Migraine Susceptibility in an Iranian Population.” Journal of Pain Research, vol. 11, 2018, pp. 145–49. PubMed, https://doi.org/10.2147/JPR.S152930.
Swartz, Michael D., et al. “Investigating Multiple Candidate Genes and Nutrients in the Folate Metabolism Pathway to Detect Genetic and Nutritional Risk Factors for Lung Cancer.” PLoS ONE, vol. 8, no. 1, Jan. 2013, p. e53475. PubMed Central, https://doi.org/10.1371/journal.pone.0053475.
Tanwar, Himani, et al. “A Computational Approach to Identify the Biophysical and Structural Aspects of Methylenetetrahydrofolate Reductase (MTHFR) Mutations (A222V, E429A, and R594Q) Leading to Schizophrenia.” Advances in Protein Chemistry and Structural Biology, vol. 108, 2017, pp. 105–25. PubMed, https://doi.org/10.1016/bs.apcsb.2017.01.007.
Thomsen, Liv Cecilie V., et al. “The Antihypertensive MTHFR Gene Polymorphism Rs17367504-G Is a Possible Novel Protective Locus for Preeclampsia.” Journal of Hypertension, vol. 35, no. 1, Jan. 2017, pp. 132–39. PubMed, https://doi.org/10.1097/HJH.0000000000001131.
Tomaszewski, Maciej, et al. “GENETIC ARCHITECTURE OF AMBULATORY BLOOD PRESSURE IN THE GENERAL POPULATION – INSIGHTS FROM CARDIOVASCULAR GENE-CENTRIC ARRAY.” Hypertension, vol. 56, no. 6, Dec. 2010, pp. 1069–76. PubMed Central, https://doi.org/10.1161/HYPERTENSIONAHA.110.155721.
Troesch, Barbara, et al. “Potential Links between Impaired One-Carbon Metabolism Due to Polymorphisms, Inadequate B-Vitamin Status, and the Development of Alzheimer’s Disease.” Nutrients, vol. 8, no. 12, Dec. 2016, p. 803. PubMed Central, https://doi.org/10.3390/nu8120803.
Wan, Lin, et al. “Age Matters: An Atypical Association Between Polymorphism of MTHFR and Clinical Phenotypes in Children with Schizophrenia.” Journal of Molecular Neuroscience: MN, vol. 69, no. 3, Nov. 2019, pp. 485–93. PubMed, https://doi.org/10.1007/s12031-019-01382-0.
Wang, Han, et al. “Association of Tagging SNPs in the MTHFR Gene with Risk of Type 2 Diabetes Mellitus and Serum Homocysteine Levels in a Chinese Population.” Disease Markers, vol. 2014, 2014, p. 725731. PubMed Central, https://doi.org/10.1155/2014/725731.
—. “Association of Tagging SNPs in the MTHFR Gene with Risk of Type 2 Diabetes Mellitus and Serum Homocysteine Levels in a Chinese Population.” Disease Markers, vol. 2014, 2014, p. 725731. PubMed Central, https://doi.org/10.1155/2014/725731.
Zhang, Donghong, et al. “Elevated Homocysteine Level and Folate Deficiency Associated with Increased Overall Risk of Carcinogenesis: Meta-Analysis of 83 Case-Control Studies Involving 35,758 Individuals.” PloS One, vol. 10, no. 5, 2015, p. e0123423. PubMed, https://doi.org/10.1371/journal.pone.0123423.
Zhang, Sheng, et al. “Association between Methylenetetrahydrofolate Reductase Tagging Polymorphisms and Susceptibility of Hepatocellular Carcinoma: A Case-Control Study.” Bioscience Reports, vol. 39, no. 11, Nov. 2019, p. BSR20192517. PubMed, https://doi.org/10.1042/BSR20192517.
Zhou, Bao-Sheng, et al. “Tagging SNPs in the MTHFR Gene and Risk of Ischemic Stroke in a Chinese Population.” International Journal of Molecular Sciences, vol. 15, no. 5, May 2014, pp. 8931–40. PubMed, https://doi.org/10.3390/ijms15058931.
—. “Tagging SNPs in the MTHFR Gene and Risk of Ischemic Stroke in a Chinese Population.” International Journal of Molecular Sciences, vol. 15, no. 5, May 2014, pp. 8931–40. PubMed, https://doi.org/10.3390/ijms15058931.
Zhou, Xinyue, et al. “The SNP Rs4846048 of MTHFR Enhances the Cervical Cancer Risk through Association with MiR-522: A Preliminary Report.” Molecular Genetics & Genomic Medicine, vol. 8, no. 1, Jan. 2020, p. e1055. PubMed, https://doi.org/10.1002/mgg3.1055.