Key takeaways:
- Nattokinase and lumbrokinase are natural enzymes studied for dissolving blood clots and microclots.
- This article covers the mechanisms of action, safety, benefits, genetics, and compares the two supplements.
- Genetic variants related to clotting potentially point to greater benefits from nattokinase.
Talk with your doctor for personalized medical advice about whether natural fibrinolytics are right for you. The information provided here is for educational purposes only and is based on a selection of peer-reviewed studies.
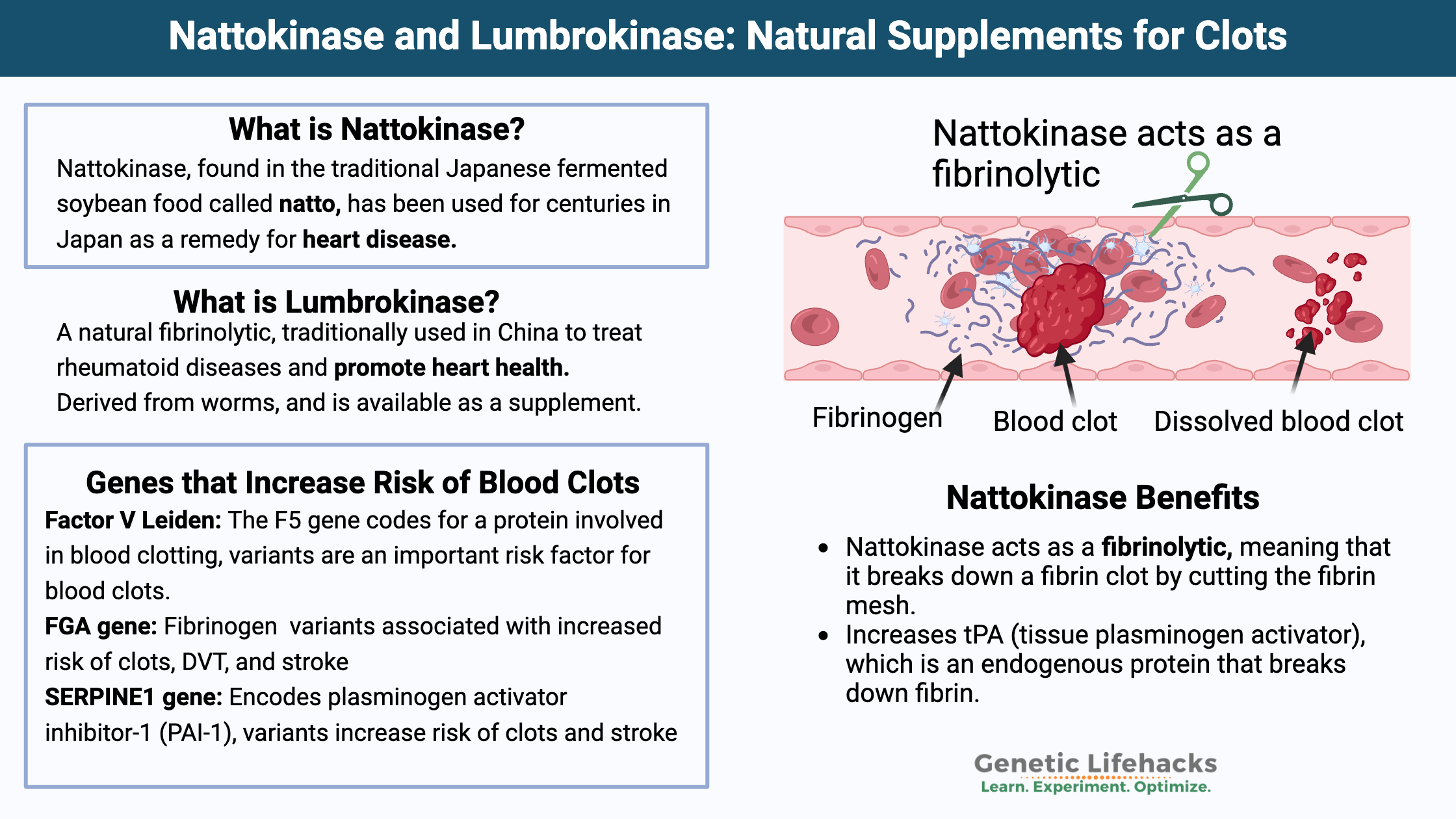
What is Nattokinase?
Nattokinase is a powerful serine protease enzyme, which means it is an enzyme that breaks apart the bonds in certain proteins. It is produced by the bacteria Bacillus subtilis natto, which is found in the traditional Japanese fermented soybean food called natto. Interestingly, natto has been used for centuries in Japan as a remedy for heart disease.[ref]
Nattokinase acts as a fibrinolytic. It means that it breaks down a fibrin clot by cutting the fibrin mesh.[ref] Recent animal research also shows that nattokinase has anti-inflammatory properties and reduces inflammation caused by lipopolysaccharide (LPS) found on bacteria.[ref]
Let’s dig into how this works and when it could be important…
Coagulation: How does blood clot?
Let me give you some quick background on how clots are made so that you can understand the process of breaking down the clot.
Coagulation, or clotting, begins very quickly when the lining of the blood vessel, the endothelium, is injured. Exposure to the collagen in the sub-endothelium of the blood vessels triggers platelet changes, causing them to clump together at the injury. At the same time, coagulation factors such as factor VII and tissue factor start forming fibrin strands, which bind together and strengthen the clot.
Fibrin is a fibrous protein made when fibrinogen encounters thrombin. Fibrinogen is a protein made in the liver that circulates in the bloodstream, ready to be converted to fibrin in an instant when needed. Thrombin is an enzyme that catalyzes the reaction causing fibrinogen to turn into insoluble fibrin strands. The gene that encodes thrombin is the F2 (factor 2) gene.
Related article: F2 and prothrombin variants
Here’s a graphical overview of the clotting process, starting with platelet activation:
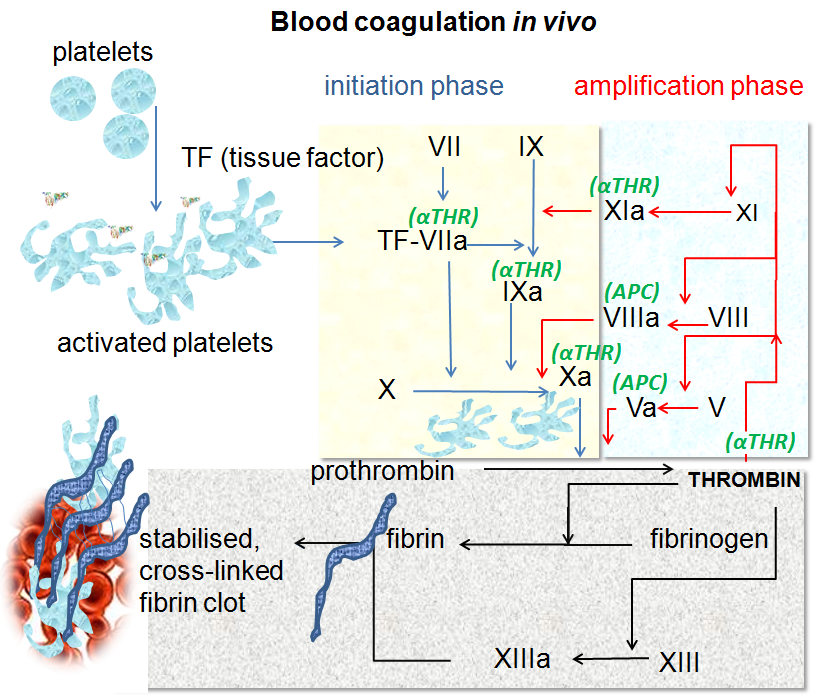
The clotting process eventually forms a stable blood clot, and then it kind of starts all over again. Clots are partly broken down and reformed continually until the injury is resolved.
Fibrinolysis: breaking down clots
Blood clots break down by a process called fibrinolysis, a term that means to cut up fibrin. Plasmin is the enzyme the body makes to break down fibrin in clots. But it isn’t as simple as just having plasmin come along to cut the fibrin. Instead, a series of actions takes place to activate plasmin. (See fibronogen genes in the genotype report section below.)
Plasminogen is the inactive form of plasmin. It is produced in the liver and circulates until it is needed. When plasminogen is converted by either tissue plasminogen activator (tPA) or urokinase, it becomes plasmin.
Here’s a visual overview of fibrinolysis, the process of breaking down clots:
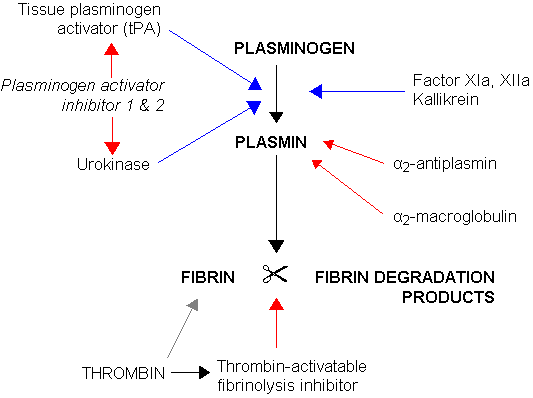
Plasminogen activator inhibitor 1 (PAI-1) regulates fibrinolytic activity in the clotting cascade. PAI-1 regulates the formation of plasmin by inhibiting tPA or urokinase. (See genotype report below for PAI-1 variants)
The breakdown of fibrin creates soluble parts called fibrin degradation products. D-dimer is one fibrin degradation product, and it is often measured to see how much clotting action is taking place in the body.
Clots Created by Immune System Activation:
Coagulation is used in the body for more than just injuries to blood vessels. Pathogens (viruses, bacteria) can be physically trapped in blood clots, and platelets play an active role in the immune system.
In addition to their role in plugging leaks in blood vessels, platelets express pathogen recognition receptors on their cell membranes. These receptors can trigger platelet activation when a virus or bacteria circulates in the bloodstream.
Platelets are created and destroyed constantly. We create about 100 billion platelets each day, and they have a lifespan of 7-10 days. They circulate through the bloodstream as tiny cells, but when activated, they undergo massive spreading and can bind to other platelets or white blood cells. Additionally, they can bind to and sequester anything identified as a pathogen (bacteria, viruses, other particles). Finally, platelets can also release antimicrobial payloads to destroy pathogens.[ref]
Covid, Spike protein, and Clots:
Access this content:
An active subscription is required to access this content.
Related Articles and Topics:
Coronary Artery Disease: Genetic Susceptibility to Heart Disease
References:
Bhatt, Prakash Chandra, et al. “Development of Surface-Engineered PLGA Nanoparticulate-Delivery System of Tet1-Conjugated Nattokinase Enzyme for Inhibition of Aβ40 Plaques in Alzheimer’s Disease.” International Journal of Nanomedicine, vol. 12, 2017, p. 8749. www.ncbi.nlm.nih.gov, https://doi.org/10.2147/IJN.S144545.
Cappelletto, Ambra, et al. SARS-CoV-2 Spike Protein Activates TMEM16F-Mediated Platelet pro-Coagulant Activity. bioRxiv, 16 Dec. 2021, p. 2021.12.14.472668. bioRxiv, https://www.biorxiv.org/content/10.1101/2021.12.14.472668v2.
Chen, Hongjie, et al. “Nattokinase: A Promising Alternative in Prevention and Treatment of Cardiovascular Diseases.” Biomarker Insights, vol. 13, July 2018, p. 1177271918785130. PubMed Central, https://doi.org/10.1177/1177271918785130.
Cooperman, Tod, and M.D. “Nattokinase Supplements Review & Top Picks.” ConsumerLab.Com, https://www.consumerlab.com/reviews/nattokinase-supplement-review/nattokinase/. Accessed 12 Nov. 2025.
Fadl, Nn, et al. “Serrapeptase and Nattokinase Intervention for Relieving Alzheimer’s Disease Pathophysiology in Rat Model.” Human & Experimental Toxicology, vol. 32, no. 7, July 2013, pp. 721–35. DOI.org (Crossref), https://doi.org/10.1177/0960327112467040.
Feng, Rui, et al. “Preparation and Toxicity Evaluation of a Novel Nattokinase-Tauroursodeoxycholate Complex.” Asian Journal of Pharmaceutical Sciences, vol. 13, no. 2, Mar. 2018, p. 173. www.ncbi.nlm.nih.gov, https://doi.org/10.1016/j.ajps.2017.11.001.
Gallelli, Giuseppe, et al. “Data Recorded in Real Life Support the Safety of Nattokinase in Patients with Vascular Diseases.” Nutrients, vol. 13, no. 6, June 2021. www.ncbi.nlm.nih.gov, https://doi.org/10.3390/nu13062031.
Gayatri, Anggi, et al. “A Clinical Trial on Biological Half Life of Bioactive Protein from Lumbricus Rubellus, DLBS1033 in Healthy Volunteers.” Acta Medica Indonesiana, vol. 50, no. 3, July 2018, pp. 208–14.
Guo, Haiyu, et al. “Comparative Anti-Thrombotic Activity and Haemorrhagic Adverse Effect of Nattokinase and Tissue-Type Plasminogen Activator.” Food Science and Biotechnology, vol. 28, no. 5, Oct. 2019, p. 1535. www.ncbi.nlm.nih.gov, https://doi.org/10.1007/s10068-019-00580-1.
Guo, Li, and Matthew T. Rondina. “The Era of Thromboinflammation: Platelets Are Dynamic Sensors and Effector Cells During Infectious Diseases.” Frontiers in Immunology, vol. 10, Sept. 2019, p. 2204. PubMed Central, https://doi.org/10.3389/fimmu.2019.02204.
Hsia, Chien-Hsun, et al. “Nattokinase Decreases Plasma Levels of Fibrinogen, Factor VII, and Factor VIII in Human Subjects.” Nutrition Research (New York, N.Y.), vol. 29, no. 3, Mar. 2009, pp. 190–96. PubMed, https://doi.org/10.1016/j.nutres.2009.01.009.
Jensen, Gitte S., et al. “Consumption of Nattokinase Is Associated with Reduced Blood Pressure and von Willebrand Factor, a Cardiovascular Risk Marker: Results from a Randomized, Double-Blind, Placebo-Controlled, Multicenter North American Clinical Trial.” Integrated Blood Pressure Control, vol. 9, 2016, p. 95. www.ncbi.nlm.nih.gov, https://doi.org/10.2147/IBPC.S99553.
Koupenova, Milka, et al. “SARS-CoV-2 Initiates Programmed Cell Death in Platelets.” Circulation Research, vol. 129, no. 6, Sept. 2021, pp. 631–46. PubMed Central, https://doi.org/10.1161/CIRCRESAHA.121.319117.
Kurosawa, Yuko, et al. “A Single-Dose of Oral Nattokinase Potentiates Thrombolysis and Anti-Coagulation Profiles.” Scientific Reports, vol. 5, 2015. www.ncbi.nlm.nih.gov, https://doi.org/10.1038/srep11601.
Lai, Chao-Hung, et al. “Lumbrokinase from Earthworm Extract Ameliorates Second-Hand Smoke-Induced Cardiac Fibrosis.” Environmental Toxicology, vol. 30, no. 10, Sept. 2015, pp. 1216–25. PubMed, https://doi.org/10.1002/tox.21993.
Pezzotta, Anna, et al. “Mesenchymal Stromal Cell Secretome Reduces Lung Injury and Thrombo-Inflammation Induced by SARS-CoV-2 Spike Protein.” Stem Cell Research & Therapy, vol. 16, July 2025, p. 324. PubMed Central, https://doi.org/10.1186/s13287-025-04472-6.
Pinzon, Rizaldy Taslim, et al. “Effect of DLBS1033 on Functional Outcomes for Patients with Acute Ischemic Stroke: A Randomized Controlled Trial.” Stroke Research and Treatment, vol. 2021, Apr. 2021, p. 5541616. PubMed Central, https://doi.org/10.1155/2021/5541616.
Ramachandran, Lintu, et al. “Nattokinase-Associated Hemoperitoneum in an Elderly Woman.” Cureus, vol. 13, no. 12, p. e20074. PubMed Central, https://doi.org/10.7759/cureus.20074. Accessed 23 Feb. 2022.
Ren, N. N., et al. “[A clinical study on the effect of nattokinase on carotid artery atherosclerosis and hyperlipidaemia].” Zhonghua Yi Xue Za Zhi, vol. 97, no. 26, July 2017, pp. 2038–42. PubMed, https://doi.org/10.3760/cma.j.issn.0376-2491.2017.26.005.
Suzuki, Yasuhiro, et al. “Dietary Supplementation with Fermented Soybeans Suppresses Intimal Thickening.” Nutrition, vol. 19, no. 3, Mar. 2003, pp. 261–64. ScienceDirect, https://doi.org/10.1016/S0899-9007(02)00853-5.
Tjandrawinata, R. R., et al. “The Safety and Tolerability of Lumbrokinase DLBS1033 in Healthy Adult Subjects.” Drug Research, vol. 66, no. 6, June 2016, pp. 293–99. PubMed, https://doi.org/10.1055/s-0035-1569406.
Tjandrawinata, Raymond R, et al. “Bioactive Protein Fraction DLBS1033 Containing Lumbrokinase Isolated from Lumbricus Rubellus: Ex Vivo, in Vivo, and Pharmaceutic Studies.” Drug Design, Development and Therapy, vol. 8, Sept. 2014, pp. 1585–93. PubMed Central, https://doi.org/10.2147/DDDT.S66007.
Wang, Yi-Hsin, Ke-Min Chen, et al. “Lumbrokinase Attenuates Myocardial Ischemia-Reperfusion Injury by Inhibiting TLR4 Signaling.” Journal of Molecular and Cellular Cardiology, vol. 99, Oct. 2016, pp. 113–22. PubMed, https://doi.org/10.1016/j.yjmcc.2016.08.004.
Wang, Yi-Hsin, Shun-An Li, et al. “Sirt1 Activation by Post-Ischemic Treatment With Lumbrokinase Protects Against Myocardial Ischemia-Reperfusion Injury.” Frontiers in Pharmacology, vol. 9, 2018, p. 636. PubMed, https://doi.org/10.3389/fphar.2018.00636.
Weng, Yunqi, et al. “Nattokinase: An Oral Antithrombotic Agent for the Prevention of Cardiovascular Disease.” International Journal of Molecular Sciences, vol. 18, no. 3, Feb. 2017, p. 523. PubMed Central, https://doi.org/10.3390/ijms18030523.
Zhang, Fuming, et al. “Interactions between Nattokinase and Heparin/GAGs.” Glycoconjugate Journal, vol. 32, no. 9, Dec. 2015, p. 695. www.ncbi.nlm.nih.gov, https://doi.org/10.1007/s10719-015-9620-8.