Minerals, known as trace elements or micronutrients, play an essential role in brain development as well as neurotransmitter levels and cognitive function in adults.
The idea of looking at your genes and knowing that you need more of a certain mineral to optimize mood is tantalizing. The reality, though, is that genetic variants may be more of a fine-tuning or just part of the picture. Often, understanding your genes can give you a starting point, but you may find that you need to experiment with diet and/or supplements to see what works for you.
Keep in mind that you may not need to take a supplement forever. You may find that once you’ve restored your levels, you don’t need it as often – or that dialing in your dietary intake may be sufficient.
Genetic variants in genes related to mineral absorption or transport can help you know what to test or try first.
Let’s dive into the research on magnesium, copper, zinc, lithium, manganese, and iron – along with the genetic connections to neurocognitive function.
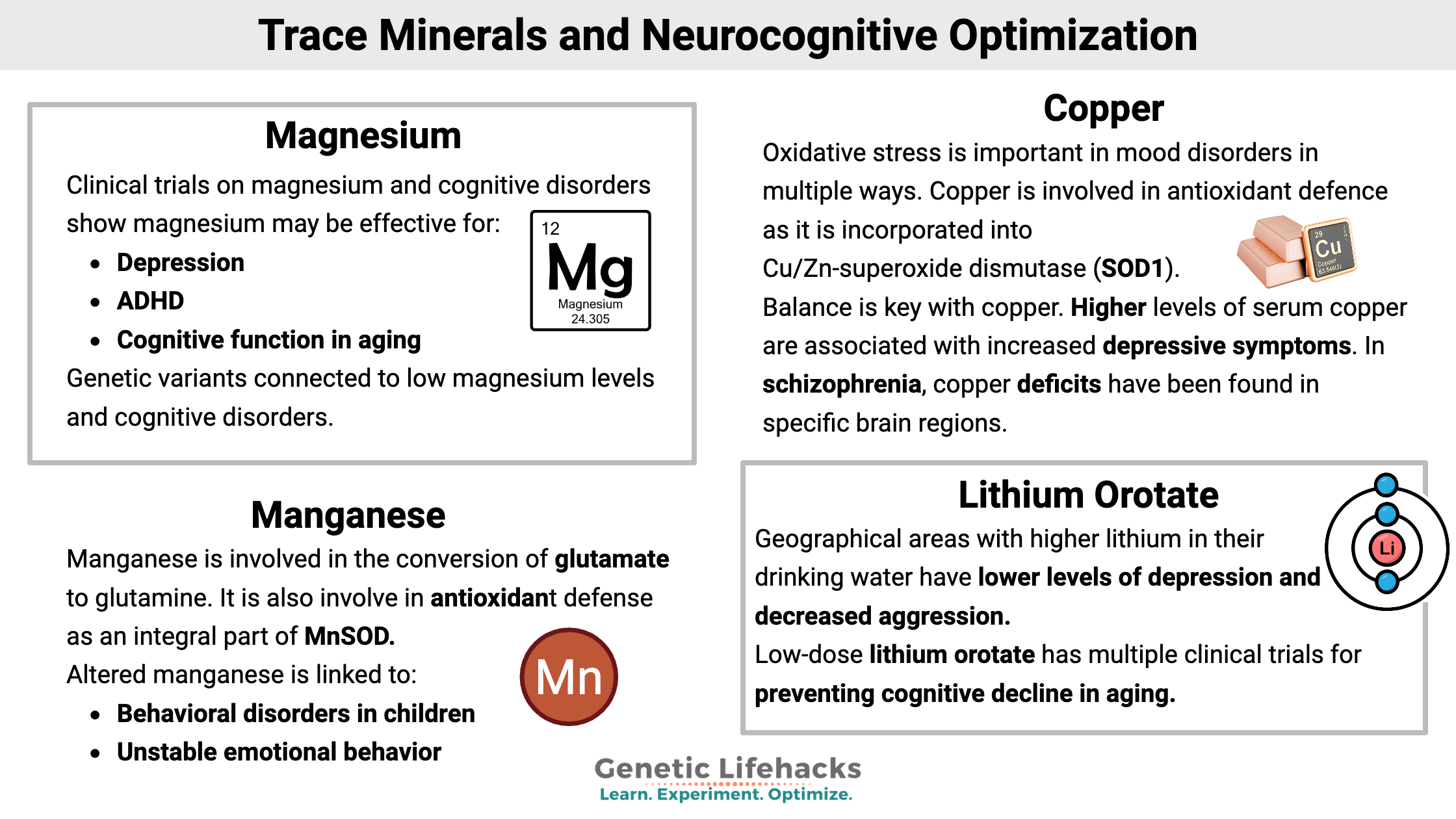
Studies link magnesium supplementation to improving different neurocognitive and psychological conditions, and you may see online headlines claiming that magnesium can cure depression, ADHD, etc. However, magnesium is unlikely to be a panacea for everyone.
Genetic variants can point to whether you are more likely to be deficient in magnesium, especially if your diet is borderline to low in this mineral. The only way to know your levels for sure is to get a blood test done, but adding in more magnesium-rich foods or a low-dose supplemental magnesium is a low-cost intervention with few drawbacks.
The US RDA for magnesium is 420 mg/day for adult males and 320 mg/day for women. Good dietary sources of magnesium include pumpkin seeds, chia seeds, almonds, spinach, cashews, peanuts, and black beans. [ref]
What happens with too much magnesium? Well, an excess of magnesium is what is used to prep for a colonoscopy… You get the picture.
Genetic variants make us all unique, including our need for individual micronutrients. Dialing in your micronutrient intake may help give you the baseline you need for optimal neurocognitive function.
Keep in mind that your need for different nutrients can fluctuate depending on stress, environmental exposure, and lifestyle factors. You may find a supplemental micronutrient very effective at certain times, but it may not be something that you need to take long-term. If you are going to supplement with a mineral for a long period of time, talk with your doctor about how often you should test your levels to make sure you’re in the right range. If you are looking for professional help with testing and optimizing, check out the Genetic Lifehacks PRO members directory.
Alemany, S., et al. “Interaction between Airborne Copper Exposure and ATP7B Polymorphisms on Inattentiveness in Scholar Children.” International Journal of Hygiene and Environmental Health, vol. 220, no. 1, Jan. 2017, pp. 51–56. PubMed, https://doi.org/10.1016/j.ijheh.2016.10.010.
Anagianni, S., and K. Tuschl. “Genetic Disorders of Manganese Metabolism.” Current Neurology and Neuroscience Reports, vol. 19, no. 6, May 2019, p. 33. Springer Link, https://doi.org/10.1007/s11910-019-0942-y.
Aron, Liviu, et al. “Lithium Deficiency and the Onset of Alzheimer’s Disease.” Nature, vol. 645, no. 8081, Sept. 2025, pp. 712–21. www.nature.com, https://doi.org/10.1038/s41586-025-09335-x.
Astorino, Maria Francesca, et al. “The Multifaceted Etiology of Mental Disorders With a Focus on Trace Elements, a Review of Recent Literature.” American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, vol. 198, no. 8, Dec. 2025, pp. 168–89. DOI.org (Crossref), https://doi.org/10.1002/ajmg.b.33045.
Baj, Jacek, et al. “Consequences of Disturbing Manganese Homeostasis.” International Journal of Molecular Sciences, vol. 24, no. 19, Oct. 2023, p. 14959. PubMed Central, https://doi.org/10.3390/ijms241914959.
Briggs, Kristi, et al. “Crohn’s Disease-Associated Pathogenic Mutation in the Manganese Transporter ZIP8 Shifts the Ileal and Rectal Mucosal Microbiota Implicating Aberrant Bile Acid Metabolism.” Inflammatory Bowel Diseases, vol. 30, no. 8, Aug. 2024, pp. 1379–88. PubMed, https://doi.org/10.1093/ibd/izae003.
Cao, Xiyu, et al. “TRPM6 and TRPM7 Genetic Polymorphisms, Dietary Magnesium, Plasma Magnesium, and Gestational Diabetes Mellitus.” The Journal of Nutritional Biochemistry, vol. 146, Dec. 2025, p. 110067. ScienceDirect, https://doi.org/10.1016/j.jnutbio.2025.110067.
Carrera, Noa, et al. “Association Study of Nonsynonymous Single Nucleotide Polymorphisms in Schizophrenia.” Biological Psychiatry, vol. 71, no. 2, Jan. 2012, pp. 169–77. PubMed, https://doi.org/10.1016/j.biopsych.2011.09.032.
Casanova, Francesco, et al. “Predictors of MRI-Estimated Brain Iron Deposition in Dementia and Parkinson’s Disease-Associated Subcortical Regions: Genetic and Observational Analysis in UK Biobank.” Journal of Alzheimer’s Disease: JAD, vol. 108, no. 1, Nov. 2025, pp. 107–18. PubMed, https://doi.org/10.1177/13872877251375432.
Chen, Jinhua, et al. “The Emerging Role of Copper in Depression.” Frontiers in Neuroscience, vol. 17, Aug. 2023. Frontiers, https://doi.org/10.3389/fnins.2023.1230404.
———. “The Emerging Role of Copper in Depression.” Frontiers in Neuroscience, vol. 17, Aug. 2023. Frontiers, https://doi.org/10.3389/fnins.2023.1230404.
Claus Henn, Birgit, et al. “Associations of Iron Metabolism Genes with Blood Manganese Levels: A Population-Based Study with Validation Data from Animal Models.” Environmental Health, vol. 10, Nov. 2011, p. 97. PubMed Central, https://doi.org/10.1186/1476-069X-10-97.
Copper in Drinking Water. https://www.health.wa.gov.au/Articles/A_E/Copper-in-drinking-water. Accessed 13 Jan. 2026.
Costas, Javier. “The Highly Pleiotropic Gene SLC39A8 as an Opportunity to Gain Insight into the Molecular Pathogenesis of Schizophrenia.” American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics, vol. 177, no. 2, Mar. 2018, pp. 274–83. PubMed, https://doi.org/10.1002/ajmg.b.32545.
Desai, Vishal, and Stephen G Kaler. “Role of Copper in Human Neurological Disorders1.” The American Journal of Clinical Nutrition, vol. 88, no. 3, Sept. 2008, pp. 855S-858S. ScienceDirect, https://doi.org/10.1093/ajcn/88.3.855S.
Ding, Jun, and Yi Zhang. “Associations of Dietary Copper, Selenium, and Manganese Intake With Depression: A Meta-Analysis of Observational Studies.” Frontiers in Nutrition, vol. 9, Mar. 2022. Frontiers, https://doi.org/10.3389/fnut.2022.854774.
Dorrego, María Flavia, et al. “A Randomized, Double-Blind, Crossover Study of Methylphenidate and Lithium in Adults with Attention-Deficit/Hyperactivity Disorder: Preliminary Findings.” The Journal of Neuropsychiatry and Clinical Neurosciences, vol. 14, no. 3, 2002, pp. 289–95. PubMed, https://doi.org/10.1176/jnp.14.3.289.
Feifel, D., and C. W. Young. “Iron Overload among a Psychiatric Outpatient Population.” The Journal of Clinical Psychiatry, vol. 58, no. 2, Feb. 1997, pp. 74–78. PubMed, https://doi.org/10.4088/jcp.v58n0204.
Fortinguerra, Stefano, et al. “Pharmacogenomic Characterization in Bipolar Spectrum Disorders.” Pharmaceutics, vol. 12, no. 1, Dec. 2019, p. 13. PubMed Central, https://doi.org/10.3390/pharmaceutics12010013.
Fu, Chuan-Yi, et al. “Increased Risk of Post-Stroke Epilepsy in Chinese Patients with a TRPM6 Polymorphism.” Neurological Research, vol. 41, no. 4, Apr. 2019, pp. 378–83. PubMed, https://doi.org/10.1080/01616412.2019.1568755.
Gale, Jenna, and Elias Aizenman. “The Physiological and Pathophysiological Roles of Copper in the Nervous System.” The European Journal of Neuroscience, vol. 60, no. 1, July 2024, pp. 3505–43. PubMed Central, https://doi.org/10.1111/ejn.16370.
Giotakos, Orestis, George Tsouvelas, et al. “A Negative Association between Lithium in Drinking Water and the Incidences of Homicides, in Greece.” Biological Trace Element Research, vol. 164, no. 2, Apr. 2015, pp. 165–68. PubMed, https://doi.org/10.1007/s12011-014-0210-6.
Giotakos, Orestis, Paul Nisianakis, et al. “Lithium in the Public Water Supply and Suicide Mortality in Greece.” Biological Trace Element Research, vol. 156, nos. 1–3, Dec. 2013, pp. 376–79. PubMed, https://doi.org/10.1007/s12011-013-9815-4.
Haile, Colin N., et al. “Pharmacogenetic Treatments for Drug Addiction.” The American Journal of Drug and Alcohol Abuse, vol. 35, no. 3, 2009, pp. 161–77. PubMed Central, https://doi.org/10.1080/00952990902825447.
———. “Pharmacogenetic Treatments for Drug Addiction.” The American Journal of Drug and Alcohol Abuse, vol. 35, no. 3, 2009, pp. 161–77. PubMed Central, https://doi.org/10.1080/00952990902825447.
Hashmi, Aisha Nasir, et al. “Association of Dopamine β-Hydroxylase Polymorphism Rs1611115 and Serum Levels with Psychiatric Disorders in Pakistani Population.” International Journal of Neuroscience, vol. 134, no. 6, June 2024, pp. 551–59. DOI.org (Crossref), https://doi.org/10.1080/00207454.2022.2126774.
Heidari, M, et al. “Brain Iron Accumulation Affects Myelin-Related Molecular Systems Implicated in a Rare Neurogenetic Disease Family with Neuropsychiatric Features.” Molecular Psychiatry, vol. 21, no. 11, Nov. 2016, pp. 1599–607. PubMed Central, https://doi.org/10.1038/mp.2015.192.
Hemamy, Mostafa, et al. “The Effect of Vitamin D and Magnesium Supplementation on the Mental Health Status of Attention-Deficit Hyperactive Children: A Randomized Controlled Trial.” BMC Pediatrics, vol. 21, no. 1, Apr. 2021, p. 178. PubMed, https://doi.org/10.1186/s12887-021-02631-1.
Hess, Mark W., et al. “Common Single Nucleotide Polymorphisms in Transient Receptor Potential Melastatin Type 6 Increase the Risk for Proton Pump Inhibitor-Induced Hypomagnesemia: A Case-Control Study.” Pharmacogenetics and Genomics, vol. 27, no. 3, Mar. 2017, pp. 83–88. PubMed, https://doi.org/10.1097/FPC.0000000000000259.
Huang, Dong, et al. “Association between Serum Copper, Zinc, and Selenium Concentrations and Depressive Symptoms in the US Adult Population, NHANES (2011–2016).” BMC Psychiatry, vol. 23, no. 1, July 2023, p. 498. Springer Link, https://doi.org/10.1186/s12888-023-04953-z.
Laudisio, Alice, et al. “Use of Proton-Pump Inhibitors Is Associated with Depression: A Population-Based Study.” International Psychogeriatrics, vol. 30, no. 1, Jan. 2018, pp. 153–59. ScienceDirect, https://doi.org/10.1017/S1041610217001715.
Lechpammer, Mirna, et al. “Pathology of Inherited Manganese Transporter Deficiency.” Annals of Neurology, vol. 75, no. 4, Apr. 2014, pp. 608–12. PubMed, https://doi.org/10.1002/ana.24131.
Liu, Mengfan, et al. “Insights into Manganese Superoxide Dismutase and Human Diseases.” International Journal of Molecular Sciences, vol. 23, no. 24, Dec. 2022, p. 15893. PubMed Central, https://doi.org/10.3390/ijms232415893.
Liu, Mengqing, et al. “Independent and Combined Effect of Serum Copper and Folate on Depression: Cross-Sectional Data from the NHANES 2011–2016.” Frontiers in Nutrition, vol. 11, Aug. 2024. Frontiers, https://doi.org/10.3389/fnut.2024.1389480.
McCann, Courtney J., et al. “SINGLE NUCLEOTIDE POLYMORPHISMS IN HUMAN ATP7B GENE MODIFY PROPERTIES OF ATP7B PROTEIN.” Metallomics : Integrated Biometal Science, vol. 11, no. 6, June 2019, pp. 1128–39. PubMed Central, https://doi.org/10.1039/c9mt00057g.
Mealer, Robert G., et al. “The Schizophrenia Risk Locus in SLC39A8 Alters Brain Metal Transport and Plasma Glycosylation.” Scientific Reports, vol. 10, Aug. 2020, p. 13162. PubMed Central, https://doi.org/10.1038/s41598-020-70108-9.
Moabedi, Mahdi, et al. “Magnesium Supplementation Beneficially Affects Depression in Adults with Depressive Disorder: A Systematic Review and Meta-Analysis of Randomized Clinical Trials.” Frontiers in Psychiatry, vol. 14, Dec. 2023, p. 1333261. PubMed Central, https://doi.org/10.3389/fpsyt.2023.1333261.
Moksnes, Marta R., et al. “A Genome-Wide Association Study Provides Insights into the Genetic Etiology of 57 Essential and Non-Essential Trace Elements in Humans.” Communications Biology, vol. 7, Apr. 2024, p. 432. PubMed Central, https://doi.org/10.1038/s42003-024-06101-z.
Nakata, Toru, et al. “A Missense Variant in SLC39A8 Confers Risk for Crohn’s Disease by Disrupting Manganese Homeostasis and Intestinal Barrier Integrity.” Proceedings of the National Academy of Sciences of the United States of America, vol. 117, no. 46, Nov. 2020, pp. 28930–38. PubMed Central, https://doi.org/10.1073/pnas.2014742117.
Noah, Lionel, et al. “Effect of Magnesium and Vitamin B6 Supplementation on Mental Health and Quality of Life in Stressed Healthy Adults: Post-Hoc Analysis of a Randomised Controlled Trial.” Stress and Health: Journal of the International Society for the Investigation of Stress, vol. 37, no. 5, Dec. 2021, pp. 1000–09. PubMed, https://doi.org/10.1002/smi.3051.
Office of Dietary Supplements – Copper. https://ods.od.nih.gov/factsheets/Copper-HealthProfessional/. Accessed 13 Jan. 2026.
Office of Dietary Supplements – Manganese. https://ods.od.nih.gov/factsheets/Manganese-HealthProfessional/. Accessed 13 Jan. 2026.
Ohi, Kazutaka, et al. “The Impact of the Genome-Wide Supported Variant in the Cyclin M2 Gene on Gray Matter Morphology in Schizophrenia.” Behavioral and Brain Functions: BBF, vol. 9, Oct. 2013, p. 40. PubMed, https://doi.org/10.1186/1744-9081-9-40.
Rose, Emma Jane, et al. “Effects of a Novel Schizophrenia Risk Variant Rs7914558 at CNNM2 on Brain Structure and Attributional Style.” The British Journal of Psychiatry: The Journal of Mental Science, vol. 204, no. 2, Feb. 2014, pp. 115–21. PubMed, https://doi.org/10.1192/bjp.bp.113.131359.
Rostami, Sepideh, et al. “A Randomized Clinical Trial Investigating the Impact of Magnesium Supplementation on Clinical and Biochemical Measures in COVID-19 Patients.” Virology Journal, vol. 21, no. 1, Apr. 2024, p. 91. PubMed, https://doi.org/10.1186/s12985-024-02362-6.
Rs121912625 RefSNP Report – dbSNP – NCBI. https://www.ncbi.nlm.nih.gov/snp/rs121912625#clinical_significance. Accessed 13 Jan. 2026.
Saraç, Mehmet, et al. “Magnesium-Permeable TRPM6 Polymorphisms in Patients with Meningomyelocele.” SpringerPlus, vol. 5, no. 1, 2016, p. 1703. PubMed, https://doi.org/10.1186/s40064-016-3395-7.
Schoonover, Kirsten E., et al. “Impaired Copper Transport in Schizophrenia Results in a Copper-Deficient Brain State: A New Side to the Dysbindin Story.” The World Journal of Biological Psychiatry: The Official Journal of the World Federation of Societies of Biological Psychiatry, vol. 21, no. 1, Jan. 2020, pp. 13–28. PubMed, https://doi.org/10.1080/15622975.2018.1523562.
Silberberg, Gilad, et al. “Stargazin Involvement with Bipolar Disorder and Response to Lithium Treatment.” Pharmacogenetics and Genomics, vol. 18, no. 5, May 2008, pp. 403–12. PubMed, https://doi.org/10.1097/FPC.0b013e3282f974ca.
Song, Yiqing, et al. “Common Genetic Variants of the Ion Channel Transient Receptor Potential Membrane Melastatin 6 and 7 (TRPM6 and TRPM7), Magnesium Intake, and Risk of Type 2 Diabetes in Women.” BMC Medical Genetics, vol. 10, Jan. 2009, p. 4. PubMed, https://doi.org/10.1186/1471-2350-10-4.
Squitti, Rosanna, et al. “Copper Excess in Psychiatric Disorders: A Focus on Mood Spectrum Disorders and Sex.” Journal of Trace Elements in Medicine and Biology, vol. 86, Dec. 2024, p. 127532. ScienceDirect, https://doi.org/10.1016/j.jtemb.2024.127532.
Stern, Shani, et al. “Prediction of Response to Drug Therapy in Psychiatric Disorders.” Open Biology, vol. 8, no. 5, May 2018, p. 180031. PubMed Central, https://doi.org/10.1098/rsob.180031.
Strodl, Esben, et al. “Probiotics and Magnesium Orotate for the Treatment of Major Depressive Disorder: A Randomised Double Blind Controlled Trial.” Scientific Reports, vol. 14, no. 1, Sept. 2024, p. 20841. www.nature.com, https://doi.org/10.1038/s41598-024-71093-z.
Surman, Craig, et al. “L-Threonic Acid Magnesium Salt Supplementation in ADHD: An Open-Label Pilot Study.” Journal of Dietary Supplements, vol. 18, no. 2, 2021, pp. 119–31. PubMed, https://doi.org/10.1080/19390211.2020.1731044.
Tsaltas, Eleftheria, et al. “Enhancing Effects of Chronic Lithium on Memory in the Rat.” Behavioural Brain Research, vol. 177, no. 1, Feb. 2007, pp. 51–60. PubMed, https://doi.org/10.1016/j.bbr.2006.11.003.
Turbiville, Donald, et al. “Iron Overload in an HFE Heterozygous Carrier: A Case Report and Literature Review.” Laboratory Medicine, vol. 50, no. 2, Apr. 2019, pp. 212–17. PubMed, https://doi.org/10.1093/labmed/lmy065.
Varga, Péter, et al. “The Role of Magnesium in Depression, Migraine, Alzheimer’s Disease, and Cognitive Health: A Comprehensive Review.” Nutrients, vol. 17, no. 13, July 2025, p. 2216. PubMed Central, https://doi.org/10.3390/nu17132216.
Wahlberg, Karin, et al. “Common Polymorphisms in the Solute Carrier SLC30A10 Are Associated With Blood Manganese and Neurological Function.” Toxicological Sciences, vol. 149, no. 2, Feb. 2016, pp. 473–83. PubMed Central, https://doi.org/10.1093/toxsci/kfv252.
Wahlberg, Karin E., et al. “Polymorphisms in Manganese Transporters SLC30A10 and SLC39A8 Are Associated With Children’s Neurodevelopment by Influencing Manganese Homeostasis.” Frontiers in Genetics, vol. 9, Dec. 2018, p. 664. PubMed Central, https://doi.org/10.3389/fgene.2018.00664.
———. “Polymorphisms in Manganese Transporters SLC30A10 and SLC39A8 Are Associated With Children’s Neurodevelopment by Influencing Manganese Homeostasis.” Frontiers in Genetics, vol. 9, Dec. 2018, p. 664. PubMed Central, https://doi.org/10.3389/fgene.2018.00664.
Ye, Qi, and Jonghan Kim. “Effect of Olfactory Manganese Exposure on Anxiety-Related Behavior in a Mouse Model of Iron Overload Hemochromatosis.” Environmental Toxicology and Pharmacology, vol. 40, no. 1, July 2015, pp. 333–41. ScienceDirect, https://doi.org/10.1016/j.etap.2015.06.016.
Yun, Yuhui, et al. “Cuproptosis-Related Gene – SLC31A1, FDX1 and ATP7B – Polymorphisms Are Associated with Risk of Lung Cancer.” Pharmacogenomics and Personalized Medicine, Volume 15, July 2022, pp. 733–42. DOI.org (Crossref), https://doi.org/10.2147/PGPM.S372824.
———. “Cuproptosis-Related Gene – SLC31A1, FDX1 and ATP7B – Polymorphisms Are Associated with Risk of Lung Cancer.” Pharmacogenomics and Personalized Medicine, Volume 15, July 2022, pp. 733–42. DOI.org (Crossref), https://doi.org/10.2147/PGPM.S372824.