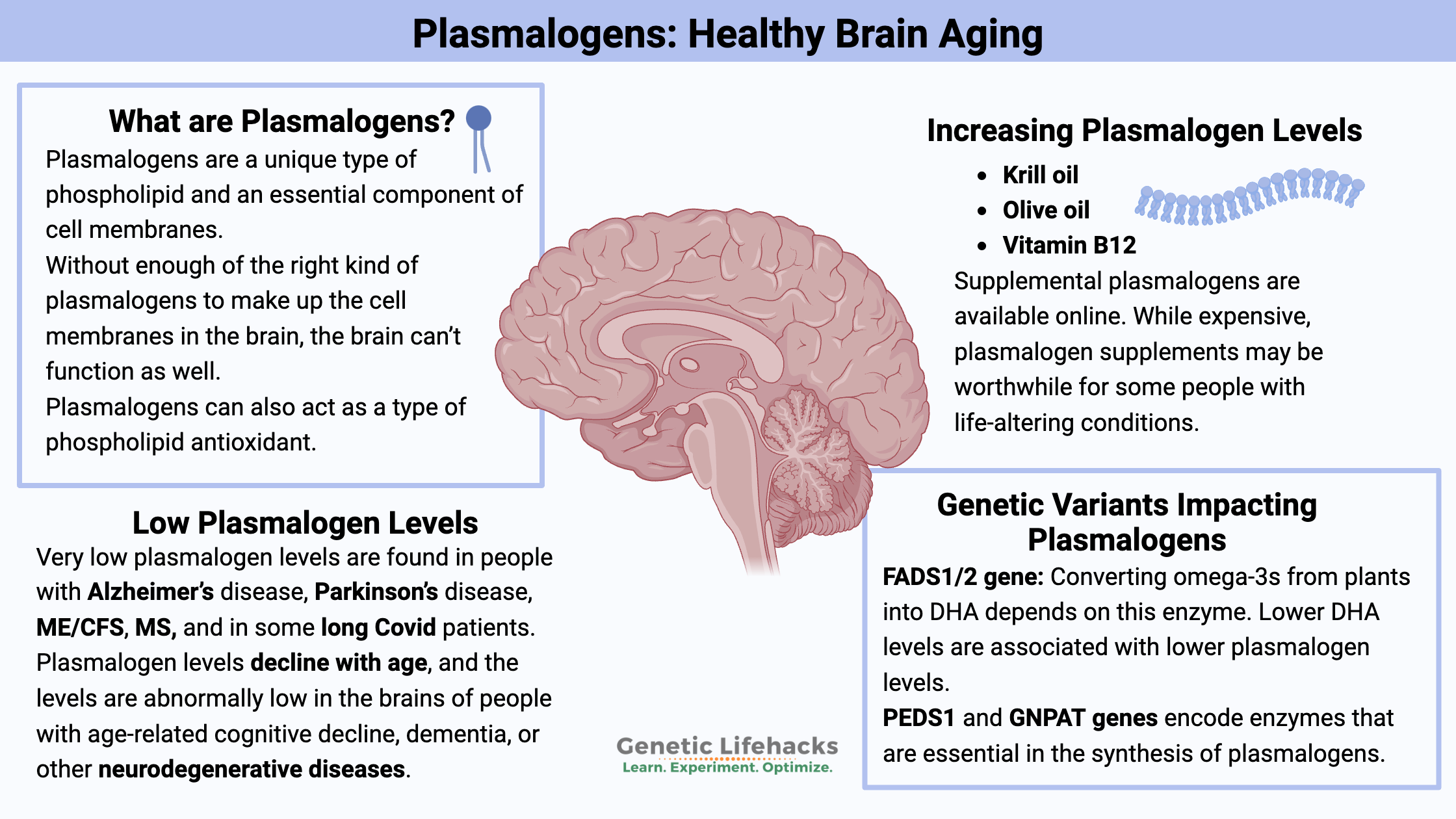
Key takeaways:
~ Plasmalogens are a type of phospholipid that make up cell membranes and can act as an antioxidant to neutralize oxidative stress.
~ Chronic oxidative stress can decrease plasmalogen levels in the brain.
~ Research suggests that plasmalogen depletion plays a causal role in Alzheimer’s disease and other neurodegenerative disorders.
~ Restoring plasmalogen levels shows promise in preventing or reversing neurological disorders.
Members will see their genotype report below, plus the natural solutions in the Lifehacks section. Join today.
What are plasmalogens?
Plasmalogens are a unique type of phospholipid and an essential component of cell membranes. Unlike typical phospholipids, plasmalogens have distinctive structural and functional characteristics that make them important in various biological processes.
Essentially, plasmalogens are made up of fatty acids along with a phosphate group, with chemical bonds that make them more flexible. The membrane surrounding each cell is composed of phospholipids, some of which are rigid due to their composition, and then plasmalogens, which are more flexible. Plasmalogens contain polyunsaturated fatty acids such as DHA.
In this article, I’ll focus primarily on the essential function of plasmalogens in the brain. Recent research shows that plasmalogens, or the lack of plasmalogens, likely play a causal role in neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and MS. I’ll also explain the studies on ME/CFS, long covid, and mood disorders.
Types of plasmalogens:
Plasmalogens differ from other phospholipids in that they have an ether linkage instead of the more common ester linkage found in other phospholipids. Specifically, this ether linkage is often a vinyl ether linkage, which is more chemically reactive. At the sn-2 position, plasmalogens typically contain polyunsaturated fatty acids, which make them fluid and flexible. In addition, plasmalogens contain a phosphate group that can be attached to various head groups such as choline or ethanolamine to form choline plasmalogen or ethanolamine plasmalogen, respectively.[ref]
In a nutshell, there are multiple subtypes of plasmalogens. In fact, about 10-20% of the phospholipids in the body are classified as plasmalogens. When plasmalogens are incorporated into the plasma membrane of the cell, they make it more fluid.
The ethanolamine plasmalogens are abundant in the brain and are thought to be important in neurodegenerative diseases. [ref] Another term for ethanolamine plasmalogens is plasmenylethanolamines.
The choline plasmalogens are found abundantly in the heart and are thought to play a role in protecting against heart disease. These are also referred to as plasmenylcholine in some studies.
What do plasmalogens do?
Plasmalogens are found in many tissues but are particularly abundant in the heart, brain, immune cells, and muscle tissue. The biosynthesis of plasmalogens takes place in the peroxisomes and endoplasmic reticulum of cells. It involves the formation of the ether bond and subsequent steps to form the complete plasmalogen molecule.
When looking at all the different roles plasmalogens play in the body, I find it helpful to keep reminding myself that plasmalogen is a general term for a class of phospholipids. Just as “fatty acid” or “cholesterol” are classes of molecules, plasmalogen refers to a type of phospholipid, not a specific molecule or protein.
Membrane Fluidity and Structure:
Plasmalogens are part of the phospholipid bilayer that surrounds cells. The unique structure of plasmalogens contributes to the fluidity and integrity of cell membranes, influencing membrane fusion and vesicle formation.
For example, the proper fluidity of the cell membrane in neurons is essential for the release of vesicles containing neurotransmitters. Without enough of the right kind of plasmalogens to make up the cell membranes in the brain, the brain can’t function as well. Animal studies show that low plasmalogen levels cause reduced neurotransmitter release in the brain due to decreased vesicle transport.[ref]
The composition of the phospholipid membrane is also important for the release of other molecules, such as cell signaling molecules and inflammatory cytokines.[ref] Additionally, plasmalogens can be taken in and used in the cell for their fatty acids. Cell membranes are constantly in flux.
Antioxidant Properties:
Plasmalogens can act as a type of phospholipid antioxidant. The vinyl ether bond in their structure can scavenge reactive oxygen species (ROS), protecting cells from oxidative stress.
The cell membranes of neurons are particularly vulnerable to oxidation, and high oxidative stress in the brain can cause neurological disorders. Plasmalogens are thought to play a critical role in being able to neutralize ROS in the brain. Essentially they act as a shield in the neuronal cell membrane, scavenging oxidized lipids. Important here, in the presence of ROS, the plasmalogens are quickly degraded. Thus, plasmalogen levels may decrease quickly as they neutralize the oxidative stress.[ref][ref][ref]
Myelin sheath:
Particularly important in brain tissue, plasmalogens make up 31-37% of the phospholipids in myelin. Myelin is the ‘insulation’ that coats the axons in fast-firing neurons in the brain and CNS. Plasmalogens are important both for the structure of the neuron and as an antioxidant defense, keeping the myelin from being damaged by ROS.[ref][ref]
Cell Signaling:
Plasmalogens are involved in cell signaling pathways. They can be precursors for various signaling molecules.[ref]
Heart Health:
While I’m not going to go into it in-depth in this article, choline plasmalogens are thought to have a role in preventing and mitigating heart diseases.
Why are plasmalogens important?
Very low plasmalogen levels are found in people with Alzheimer’s disease, Parkinson’s disease, ME/CFS, MS, and in some long Covid patients. In some people with long-term depression or bipolar disorder, low plasmalogen levels can be a factor.
Plasmalogen levels decline with age, and the levels are abnormally low in the brains of people with age-related cognitive decline, dementia, or other neurodegenerative diseases.
As a biomarker, plasmalogens hold promise for understanding and predicting certain neurological and cardiovascular diseases. There are tests in the works for plasmalogen levels as a biomarker for Alzheimer’s dieses.
Why is this potentially so exciting?
Supplementation with plasmalogens or dietary changes that increase plasmalogens may help maintain membrane integrity and fluidity in nerve cells, potentially slowing the progression of neurodegenerative diseases or preventing neurodegeneration in the first place.[ref] In addition, understanding the link between oxidative stress and low plasmalogens explains what happens when the brain is overwhelmed and reaches the tipping point into a disease state.
Plasmalogen types in the brain:
In the brain, ethanolamine plasmalogen is involved in the structure of the cell membranes. The shorter chain plasmalogens (18:1 20:1, and 22:4 fatty acids) are found in the white matter of the brain, while the longer chain plasmalogens (22:6, 20:4, and 22:4 fatty acids) are found in the gray matter.[ref]
For example, the plasmalogens that make up the myelin sheath primarily incorporate DHA or arachidonic acid.[ref] In the brain, plasmalogens act as a reservoir for these important fatty acids (DHA, AA) to be accessed when needed.[ref]
The length of the fatty acid chains in the plasmalogen is important in brain health. Fatty acids can be changed by elongation and desaturation, using the FADS1 and FADS2 enzymes. Abundant in fish oil, DHA and EPA can also be derived from plant-based omega-3 fatty acids, but many people have genetic variants that decrease that conversion. (See FADS1/2 in the genotype report section)
What causes plasmalogen levels to decrease in aging?
The level of plasmalogen in the brain decreases by about 40% from age 40 to 70.[ref] There are multiple factors that come into play here, causing both decreased synthesis of plasmalogens and increased degradation.
The synthesis of DHA (22:4) is reduced in aging, as is the incorporation of DHA into ethanolamine plasmalogen.
A second factor involved in the decline of plasmalogen levels in aging is an increased production of the enzyme that breaks down ethanolamine plasmalogens.[ref]
As an antioxidant, plasmalogen is degraded by high levels of ROS or chronic oxidative stress in the brain. Mitochondrial dysfunction causes a decrease in plasmalogen levels, and cytochrome C can break plasmalogens apart.[ref]
Plasmalogen levels are significantly decreased in the brains of Alzheimer’s Disease (AD) patients. While there is still some uncertainty as to the cause, one big clue is that brain tissue samples in AD patients show decreased peroxisomes, the organelles that synthesize plasmalogens.[ref]
One more thing to keep in mind as you read on:
Animal studies show that depleting omega-3 fatty acids causes a decrease in plasmalogen levels in the brain.[ref] There has been a fundamental shift in the fatty acid makeup of the standard diet over the past 60 years or so. Historically, people consumed a lot more omega-3 fatty acids, especially DHA and EPA, compared to their omega-6 intake. Seed oils, such as canola and soybean, are now consumed abundantly, and they contain large amounts of omega-6 oils. This has shifted our dietary ratio of omega-6:omega-3 fats significantly. Additionally, meat, dairy, and eggs traditionally contain more omega-3 fatty acids from grazing on grass, but the shift to industrialized farming has changed the composition of animal foods. When looking at plasmalogens, the relative lack of DHA could be playing a role in brain resiliency.
Neurotransmitter release:
One way that plasmalogens are important in the brain is in neurotransmitter release in neurons. Neurotransmitters are synthesized in the neuron and then packaged into vesicles. These vesicles are a phospholipid bilayer that surrounds the neurotransmitter. To release the neurotransmitter in the synapse of the neuron, the vesicles dock and fuse with the cell membrane.
Here’s an illustration showing the vesicles merging with the neuron’s cell membrane and releasing the neurotransmitters:
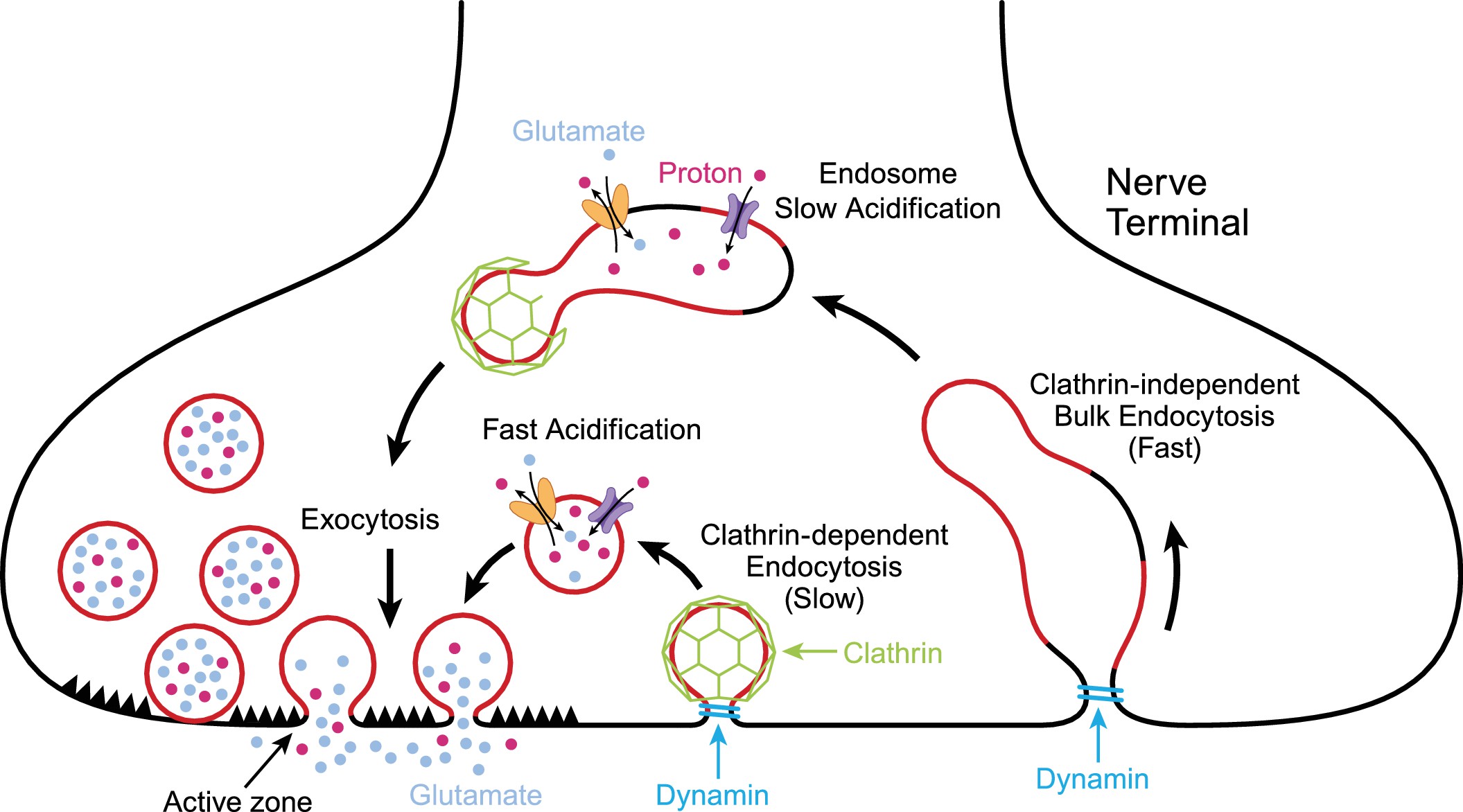
Ethanolamide plasmalogens make up part of the lipid membrane surrounding the neurotransmitters, as well as the cell membrane of the neurons. They are important in the fluidity of the membranes and being able to easily release the neurotransmitters.
GNPAT is a key enzyme in the formation of plasmalogens, and an animal model with the GNPAT gene knocked out resulted in “a more generalized reduction of neurotransmitters and aberrant neurotransmitter release”. The reduction in neurotransmitters included dopamine, serotonin, GABA, and norepinephrine.[ref]. (See genotype report for GNPAT)
Ethanolamide:
I keep writing ethanolamide plasmalogens without really explaining what “ethanolamide” means and where it comes from. One pathway that generates ethanolamide is the conversion of phosphatidylethanolamine (PE) into phosphatidylserine (PS) through a reaction with serine. The CDP-ethanolamine pathway is involved in PE synthesis. It is thought that both of these pathways can produce the ethanolamide that reacts to form ethanolamide plasmalogens. [ref]
While all of this isn’t necessary to understand in detail, the takeaway is that serine, phosphatidylserine, and choline can play a role here.
Plasmalogens and Neurodegenerative Diseases:
Let’s dive into the research on how low plasmalogen levels play a role in neurodegenerative diseases, such as Alzheimer’s and dementia, as well as chronic conditions that have mitochondrial dysfunction as a root cause.
Alzheimer’s Disease and Dementia:
Research shows that ethanolamine plasmalogen is decreased in Alzheimer’s brains. Importantly, the severity of dementia in Alzheimer’s patients correlates to the ethanolamine plasmalogen content in the cortex and hippocampus areas of the brain.
Additionally, circulating serum ethanolamine plasmalogen levels are significantly decreased in patients with dementia, and the severity of the decrease mirrors the severity of the dementia.[ref] The circulating levels mirroring the decrease in the brain may mean two things: One, measuring serum plasmalogens may help predict the risk of Alzheimer’s, and two, increasing serum plasmalogens (diet, supplements) may help to fix the problems in the brain.
Clinical trials in Japan have shown supplemental plasmalogen to be effective at improving memory in people with Alzheimer’s. It seems to be more effective in mild AD and in younger patients.[ref][ref]
One thing that goes wrong in the brain of people with AD is the build-up of amyloid-beta plaques of different lengths. In addition to all the other roles that plasmalogens play in keeping neurons healthy and functioning well, ethanolamide plasmalogen also reduces the activity of γ–secretase, which is an enzyme needed for the formation of the amyloid-beta plaque.[ref]
You may be thinking “Why haven’t I heard about plasmalogens for Alzheimer’s?” While a lot of the research on plasmalogen levels in Alzheimer’s is new, this change in lipid levels in the brain has actually been known for several decades…
A study from 1999 explains: “The total phospholipid in the frontal cortex and hippocampus decreased on a DNA basis by about 20% and this change was essentially explained by a selective decrease in phosphatidylethanolamine and phosphatidylcholine. The lower content of phosphatidylethanolamine was due to a specific decrease in the plasmalogen subclass. Phosphatidylethanolamine plasmalogen was also the only lipid exhibiting major structural modifications: a significant decrease in polyunsaturated fatty acids and oleic acid as well as a shift of the aldehyde pattern from 18:1 to 18:0.”[ref]
I’m not sure why the focus of Alzheimer’s research for the past couple of decades has been on preventing amyloid-beta formation, but there seems to be a shift recently with a lot of new research based on lipid metabolism and oxidative stress in the brain.
Interaction with APOE E4 and Alzheimer’s risk:
APOE (apolipoprotein E) is a lipid and cholesterol transport gene. People with APOE E4 alleles are at a higher risk of Alzheimer’s disease, and the APOE E2 genotype protects against Alzheimer’s. The APOE E3/E3 genotype is considered neutral in risk.
A study showed that people with APOE E4 alleles have lower plasmalogen levels, while people with APOE E2 have higher levels.[ref] This goes along with multiple studies that show that plasmalogen levels are lower in Alzheimer’s brains.[ref]
The question for a long time was whether the decreased plasmalogen levels caused AD or if Alzheimer’s causes a decrease in plasmalogen.
Cell studies, animal studies, and even a couple of clinical trials show that low plasmalogen levels are likely playing a causal role in Alzheimer’s disease. Excitingly, the studies on increasing plasmalogen show that increasing the levels prior to the onset of dementia may mitigate the risk.[ref]
Optimal plasmalogen levels can mitigate the increased APOE E4 risk in Alzheimer’s disease.[ref][ref] To put that into context, carrying one copy of APOE4 doubles your risk of Alzheimer’s, but returning your plasmalogen levels to normal cuts that back to the normal risk of Alzheimer’s.
One link specific to APOE is that ethanolamine plasmalogen plays a role in cholesterol trafficking. [ref]
ME/CFS and Long Covid:
ME/CFS stands for myalgic encephalomyelitis/chronic fatigue syndrome. It is a system-wide, chronic condition that affects many body systems. For many (most?) people, it is thought to be triggered by a viral infection.
Related article: ME/CFS genetic links
A viral infection in the brain is a big cause of neuroinflammation…
Plasmalogen levels are significantly decreased in people with ME/CFS and for some long Covid patients. One study showed decreased levels of plasmalogens, phosphatidylcholine, and sphingomyelins in patients with ME/CFS compared to an age-matched healthy control group. The plasmalogen levels were drastically reduced – more than the other changes in lipids. [ref]
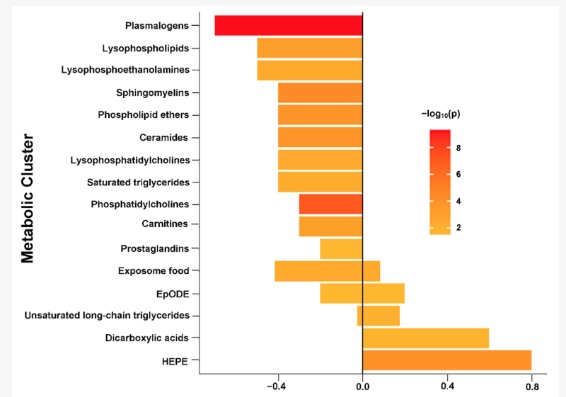
Another study on ME/CFS patients also had a similar finding of patients having uniformly low plasmalogen levels. The study looked at hundreds of metabolic markers, and the effect on ethanolamine plasmalogen levels was seen across the board in the ME/CFS patients.[ref]
Multiple Sclerosis:
Multiple Sclerosis (MS) is an autoimmune disease in which the myelin sheath surrounding neurons is attacked and damaged.
A recent study looked in-depth at the lipid levels in people with MS. The results showed that plasmalogen levels, including both ethanalomine plasmalogens and choline plasmalogens, were significantly decreased in MS patients.[ref] In patients with relapsing-remitting MS, higher plasmalogen levels were associated with lower relapse risk.[ref]
Animal models of MS show that CDP-choline may help with remyelination.[ref] Human studies on CDP-choline and on plasmalogens for MS are lacking, at this point.
Parkinson’s Disease:
Plasmalogens may also play a significant role in brain health in Parkinson’s disease. Research studies show that plasmalogen levels are decreased by 30% in people with Parkinson’s. High oxidative stress and mitochondrial function in the brain are also a part of Parkinson’s disease, leading to low plasmalogen levels. It is thought that the low plasmalogen levels then lead to a loss of cellular signaling in cortical gray matter.[ref][ref]
A small clinical trial showed that supplemental plasmalogen (purified ether phospholipids derived from scallops) reduced Parkinson’s symptoms after 24 weeks and helped to restore plasmalogen levels to normal.[ref]
Depression and Bipolar Disorder:
Neuroinflammation can play a significant role in mood disorders.
Knocking out a key gene in ethanolamine plasmalogen synthesis in mice causes depressive symptoms and memory loss.[ref] Human studies, though, show that ether-phosphodatidylcholines are altered in depression.[ref]
In a placebo-controlled trial, 2g/day of plasmalogen supplements improved mood and cognitive function in healthy college-aged study participants. The Total Mood Disturbance score improved and aggression decreased in the plasmalogen group.[ref] Note that these students weren’t patients diagnosed with a depressive disorder, so that change in mood was elevated from a fairly normal baseline.
A 2020 study showed that people with bipolar disorder 1 had significantly lower ethanolamine plasmalogen levels compared to a matched control group. The statistical significance was only seen in BPI (more severe symptoms). While plasmalogen levels are likely not the only change going on in the brain with bipolar disorder, the reduced level points towards mitochondrial dysfunction causing oxidative stress.[ref]
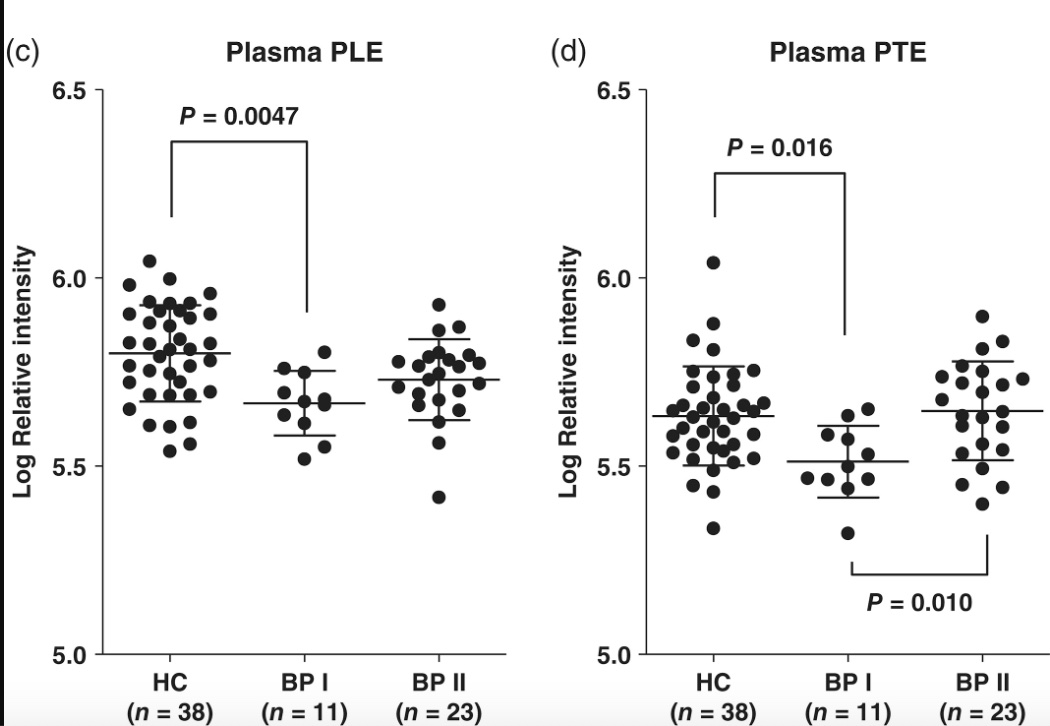
Underlying thread: Oxidative stress, low plasmalogens
All of these chronic brain-related conditions – AD, Parkinson’s, ME/CFS, long Covid, MS, and mood disorders – have elevated oxidative stress due to mitochondrial dysfunction in common, which can directly lead to the continual loss of plasmalogens. While it is exciting that supplemental plasmalogens can decrease symptoms and restore brain function, preventing mitochondrial dysfunction and oxidative stress in the first place should also be a consideration.[ref]
While I’ve focused in on a few examples of neurological conditions that rely on sufficient plasmalogen levels in the brain, I wanted to mention that I’m leaving a lot out, such as TBI, autism, ALS, motor cortex disorders, and more.[ref][ref]
Genetics and plasmalogen levels:
What happens if you don’t have sufficient production of plasmalogen? Well, rare genetic mutations can show us that it is very detrimental not to be able to produce plasmalogens.
Genes Involved in Plasmalogen Synthesis
The synthesis of plasmalogens involves several enzymes, and genes encoding these enzymes are potential sites for polymorphisms. Key enzymes include glycerone phosphate O-acyltransferase (GNPAT), alkylglycerone phosphate synthase (AGPS), and peroxisomal dihydroxyacetone phosphate acyltransferase (DHAPAT).
Researchers recently discovered that fatty acyl-CoA reducatse 1 (FAR1) is essential in the regulation of plasmalogen biosynthesis which fluctuates in response to the cellular levels of plasmalogens.[ref]
Peroxisomal Disorders
Genetic disorders that affect peroxisomes, where part of the plasmalogen synthesis occurs, can significantly impact plasmalogen levels. Examples include Zellweger syndrome, Rhizomelic chondrodysplasia punctata (RCDP), and other peroxisome biogenesis disorders. Both of these disorders cause neurological problems, such as significantly impaired cognitive function and even seizures.
These disorders often result from mutations in genes related to peroxisome formation and function, indirectly affecting plasmalogen synthesis.
Apart from rare mutations that cause peroxisome dysfunction, there are a few more common genetic variants that impact plasmalogen synthesis that will be covered in the genotype report below.
Genotype report: plasmalogens
The following genetic variants have been linked in research studies to plasmalogen levels. The research here is still pretty new, and I’m sure there will be a lot more discovered on this topic in the future.
Lifehacks:
If plasmalogens are a linchpin for brain health, what can we do to prevent the decline – or – increase levels if low?
In this section, I’m going to focus on:
- supplements that increase plasmalogen levels
- foods that contain plasmalogens
- why a ketogenic diet increases plasmalogens
- how to tamp down neuroinflammation and improve mitochondrial function
As always, talk with your doctor if you have any medical questions about whether a diet change or a supplement is right for you.
Related articles:
ADHD Genes: Exploring the Role of Genetics, Environment, and Neurochemistry in ADHD