Key takeaways:
~ Genetic mutations in cardiac muscle proteins cause hypertrophic cardiomyopathy.
~ This is one cause of sudden cardiac death, especially in young adult athletes. It is the reason sports physicals have been required since the 1980s.
~ Knowing about a hypertrophic cardiomyopathy mutation means you can get the necessary medical treatments to prevent future problems.
Hypertrophic cardiomyopathy
Cardiomyopathy is a general term for diseases affecting the heart muscle. There are several types of cardiomyopathy. Here, I’m focusing on hypertrophic cardiomyopathy.
Hypertrophic cardiomyopathy is a genetic disease caused by mutations in the genes involved in heart muscle formation. It is estimated that between 1 in 200 and 1 in 500 persons have it.[ref][ref]
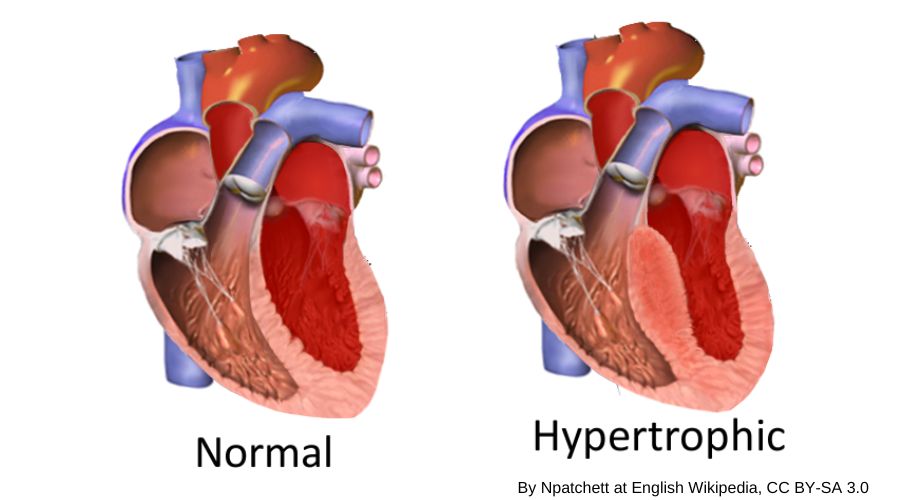
In hypertrophic cardiomyopathy, the heart muscle wall thickens. It is diagnosed when the wall of the left ventricle (left, bottom chamber) is enlarged – and – no other reasons, such as really high blood pressure or aortic stenosis, have been identified to explain the enlargement.[ref]
Often people are asymptomatic and don’t realize they have a heart problem. However, some people with hypertrophic cardiomyopathy have symptoms that include shortness of breath, angina, or heart palpitations.
Here’s an image showing the enlarged ventricle wall, which reduces the volume of blood that can be contained in the left ventricle:
What causes hypertrophic cardiomyopathy?
The functional units of muscular tissue, known as sarcomeres, enable muscle contraction and relaxation. The cardiac muscle (or myocardium) making up your heart is composed of layers of muscle. This type of muscle is specific to the heart and is slightly different from skeletal muscles.
In people with hypertrophic cardiomyopathy, the proteins that make up the cardiac muscle sarcomeres are altered.
Contractions in the heart muscle are triggered by calcium ions. In hypertrophic cardiomyopathy, the sarcomeres in the cardiac muscle aren’t functioning quite as they should due to mutations in the genes encoding the heart muscle proteins. For example, one of the genetic mutations linked to hypertrophic cardiomyopathy causes more contractility, more sensitivity to calcium ions, and less cardiac muscle relaxation.[ref]
The thickening of the heart wall can cause fibrosis which stiffens the heart muscle. Hypertrophic cardiomyopathy can also cause periodic issues with ventricular arrhythmias. Additionally, the valve regulating blood flow into another heart chamber may be partially obstructed by the thick ventricular muscle, leading to a leaky valve.[ref]
Sudden cardiac death: young athletes and hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy patients are at an increased risk of sudden cardiac death. It can occur even without structural changes in the heart.[ref]
Researchers estimate the risk of sudden cardiac death to be about 1% per year in patients with hypertrophic cardiomyopathy. However, cardiologists can help patients manage hypertrophic cardiomyopathy and reduce the risk of sudden death with appropriate medications or technology such as implanted defibrillators.
Hypertrophic cardiomyopathy is the main cause of sudden cardiac death in young adults, especially in athletes.[ref] There are other causes, of course, but hypertrophic cardiomyopathy accounts for over half of sudden cardiac deaths.
Before going into why sudden cardiac death is a risk, let me put the numbers in perspective:
1 in 300 people with hypertrophic cardiomyopathy, and a 1% risk of sudden death in those people… this can be hard to visualize and may leave you wondering if kids normally have heart-related problems on the football field.
Competitive athletes dying of sudden cardiac death due to cardiomyopathy prompted most sports organizations in the 1980s to start requiring a sports physical before playing.
Due to screenings, the risk of sudden cardiac death in student athletes is much, much lower today than it was several decades ago.
This reduction in deaths is why sports physicals are required pretty much everywhere. Parents all know the scramble to get a physical before signing up for middle school soccer or playing any sport in college.
A study of all student athletes in Minnesota showed that when sports physicals were implemented, the number of sudden cardiac deaths over a 12-year period was only 3. The researchers estimated the risk of sudden death was 1 in 500,000 athletes.[ref]
Other studies put the risk at about 1 in 200,000 student athletes per year and 1 in 100,000 when including high school, college, and professional athletes based on multiple public record sources. The numbers are similar in the US and European countries.[ref]
In Italy, a study found that from 1979-2004, a 26-year period, there were 55 sudden deaths in Italian athletes who had been screened for cardiac issues (2 per year). This was in sharp contrast to the 265 sudden deaths (or about 10/year) in athletes who had not been screened for cardiac issues.[ref]
What goes wrong? How can the heart be beating normally, day after day, and then suddenly have an electrical issue that kills you?
Pacemaker cells in the sinoatrial node in the right atrium set the heart’s rhythm. The cardiac muscle cells then contract in coordination with neighboring cells, working together to pump blood from the heart.
Ions moving in and out of the cells, including calcium, potassium, and sodium ions, produce the electrical impulses that cause heart muscle contractions. All of this happens automatically and in coordination throughout the heart.
Unfortunately, the disordered muscle cells in hypertrophic cardiomyopathy are more susceptible to ventricular arrhythmias. Research shows that cardiac ion channels are more sensitive to calcium ions in the ventricular muscle fibers of people with hypertrophic cardiomyopathy.[ref][ref]. Animal studies also point to potassium channel currents being reduced in addition to increased myofilament calcium sensitivity.[ref]
Together, the changes add up to people with hypertrophic cardiomyopathy being at increased risk for the type of ventricular rhythm issues which cause the heart to suddenly stop beating. The strain on the heart during physical activity can combine with not drinking enough and having electrolytes slightly off… leading to ventricular arrhythmia.
Genes involved:
Hundreds of rare mutations have been discovered in more than 20 genes related to cardiac muscle formation and function. These genetic mutations can cause hypertrophic cardiomyopathy.[ref]
The mutations are classified as autosomal dominant, which means a person only needs one copy of the mutation to have the effect. Most of the time, the mutation is inherited from either mom or dad. But sometimes, the mutation may be sporadic, arising through a new mutation in the embryo.[ref]
The more common (but still rare) mutations are found in the MYBPC3 and MYH7 genes.
Here’s an image showing the involvement of the different cardiac muscle proteins in how the heart contracts (CC 4.0)[ref]:
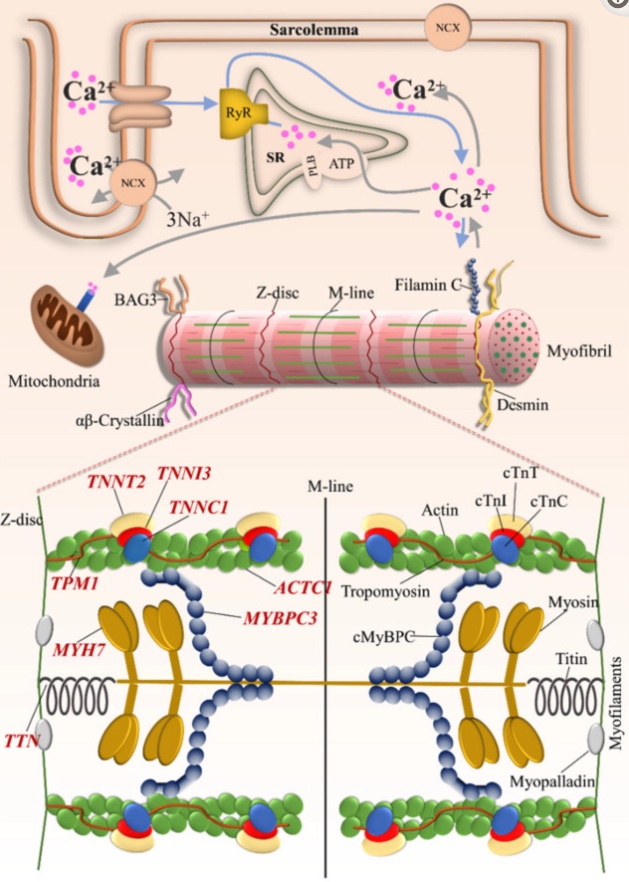
MYBPC3 (myosin-binding protein C, cardiac type) is a heart muscle-specific protein. MYBPC3 binds to myosin, actin, and titin, which are integral to heart muscle function.
Animal studies show that eliminating the MYBPC3 results in severe heart malformation – cardiac hypertrophy and altered rhythm. MYBPC3 acts as a brake on contractions in the heart.
The MYH7 gene encodes a myosin (muscle) protein found mainly in the heart. Myosins are the part of the muscle cell which binds to actin and ‘walk’ along the filament to contract the muscle.
Here’s a quick video to give you a visual of the sarcomere showing myosin, actin, and troponin:
Hypertrophic cardiomyopathy and Covid:
You may be thinking (as I was) that hypertrophic cardiomyopathy must be a big risk factor in dying of COVID-19. Well, it looks like I was wrong…
ACE2 is the receptor used by SARS-CoV2 to enter cells. In hypertrophic cardiomyopathy, the ACE2 receptor is upregulated in the heart muscle.[ref]
Doctors initially were concerned that hypertrophic cardiomyopathy may be a big mortality risk factor, but the studies don’t seem to bear that out – at least not in older adults.
A study in NY looked at 70 hypertrophic cardiomyopathy patients with positive COVID-19 tests. The average age was 62. The results showed that hypertrophic cardiomyopathy didn’t increase the risk of death or severity. Interestingly, almost a quarter of the patients were asymptomatic with SARS-CoV-2 infection.[ref]
Hypertrophic Cardiomyopathy Genotype Report
Caution. Please keep the following in mind:
- First, raw data from 23andMe and AncestryDNA isn’t guaranteed to be clinically accurate.
- Second, not all of the known mutations are listed here. 23andMe and AncestryDNA data don’t cover everything, and I’m just including the well-researched and more common mutations.
- Third, additional mutations are linked to hypertrophic cardiomyopathy, which aren’t included here.
Use this information as a heads-up to talk to your doctor. But please understand that this information isn’t definitive and can’t be used to rule out a mutation.
Members: Log in to see your data below.
Not a member? Join here.
Why is this section is now only for members? Here’s why…
Lifehacks:
If your genetic data shows one of the mutations above, talk with your doctor or cardiologist about your next steps.
Be sure your doctor understands that your genetic data was from a direct-to-consumer testing company and should be confirmed with a test from a CLIA-certified lab. For example, Invitae offers CLIA cardiomyopathy genetic testing, which is much more comprehensive information than what you can get from 23andMe or AncestryDNA.
What have I left out here? In addition to only covering the more common mutations, I have also only touched on one genetic reason for sudden cardiac death. Dilated cardiomyopathy is another type of inherited heart issue that can cause sudden cardiac death.
Beyond the rare genetic links to cardiomyopathy, there are many other, much more common genetic variants to look at for heart health.
Other heart issues to check your genes for include:
Related Articles and Topics:
Spike protein, histamine, heart rhythm:
Find out how the histamine receptors in the heart play a role in heart rhythm.
ADRA1A Receptors: Blood vessel reactions under stress:
Adrenergic receptors regulate blood flow to the periphery when you are under stress.
Small Fiber Neuropathy: Genetics, Causes, and Possible Solutions
In small fiber neuropathy, the tiniest nerve fibers break down and cause burning pain, numbness, odd sensations, or autonomic nervous system issues.
References:
Arabadjian, Milla E., et al. “COVID-19 in Adults With Hypertrophic Cardiomyopathy.” Frontiers in Cardiovascular Medicine, vol. 8, Nov. 2021, p. 745790. PubMed Central, https://doi.org/10.3389/fcvm.2021.745790.
Bos, J. Martijn, et al. “Marked Up-Regulation of ACE2 in Hearts of Patients With Obstructive Hypertrophic Cardiomyopathy: Implications for SARS-CoV-2–Mediated COVID-19.” Mayo Clinic Proceedings, vol. 95, no. 7, July 2020, pp. 1354–68. PubMed Central, https://doi.org/10.1016/j.mayocp.2020.04.028.
Corrado, Domenico, et al. “Trends in Sudden Cardiovascular Death in Young Competitive Athletes After Implementation of a Preparticipation Screening Program.” JAMA, vol. 296, no. 13, Oct. 2006, pp. 1593–601. Silverchair, https://doi.org/10.1001/jama.296.13.1593.
Emery, Michael S., and Richard J. Kovacs. “Sudden Cardiac Death in Athletes.” JACC: Heart Failure, vol. 6, no. 1, Jan. 2018, pp. 30–40. ScienceDirect, https://doi.org/10.1016/j.jchf.2017.07.014.
Flenner, Frederik, et al. “Translational Investigation of Electrophysiology in Hypertrophic Cardiomyopathy.” Journal of Molecular and Cellular Cardiology, vol. 157, Aug. 2021, pp. 77–89. PubMed Central, https://doi.org/10.1016/j.yjmcc.2021.04.009.
Hassoun, Roua, et al. “Cardiomyocyte Dysfunction in Inherited Cardiomyopathies.” International Journal of Molecular Sciences, vol. 22, no. 20, Oct. 2021, p. 11154. PubMed Central, https://doi.org/10.3390/ijms222011154.
Invitae Cardiomyopathy Comprehensive Panel | Test Catalog | Invitae. https://www.invitae.com/en/providers/test-catalog/test-02251. Accessed 5 Oct. 2022.
Liu, Hui-Ting, et al. “Screening of MYH7 Gene Mutation Sites in Hypertrophic Cardiomyopathy and Its Significance.” Chinese Medical Journal, vol. 132, no. 23, Dec. 2019, pp. 2835–41. PubMed Central, https://doi.org/10.1097/CM9.0000000000000428.
Maron, Barry J., et al. “Diagnosis and Evaluation of Hypertrophic Cardiomyopathy: JACC State-of-the-Art Review.” Journal of the American College of Cardiology, vol. 79, no. 4, Feb. 2022, pp. 372–89. ScienceDirect, https://doi.org/10.1016/j.jacc.2021.12.002.
Maron, Barry J, et al. “Prevalence of Sudden Cardiac Death during Competitive Sports Activities in Minnesota High School Athletes.” Journal of the American College of Cardiology, vol. 32, no. 7, Dec. 1998, pp. 1881–84. ScienceDirect, https://doi.org/10.1016/S0735-1097(98)00491-4.
NM_000256.3(MYBPC3):C.2373dup (p.Trp792fs) AND Cardiovascular Phenotype – ClinVar – NCBI. https://www.ncbi.nlm.nih.gov/clinvar/109795703/. Accessed 5 Oct. 2022.
NM_000257.3(MYH7):C.1207C>T (p.Arg403Trp) AND Hypertrophic Cardiomyopathy – ClinVar – NCBI. https://www.ncbi.nlm.nih.gov/clinvar/RCV000456661.1/. Accessed 5 Oct. 2022.
Nykamp, Keith, et al. “Sherloc: A Comprehensive Refinement of the ACMG–AMP Variant Classification Criteria.” Genetics in Medicine, vol. 19, no. 10, Oct. 2017, pp. 1105–17. DOI.org (Crossref), https://doi.org/10.1038/gim.2017.37.
Richards, Sue, et al. “Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology.” Genetics in Medicine, vol. 17, no. 5, May 2015, pp. 405–24. DOI.org (Crossref), https://doi.org/10.1038/gim.2015.30.
Suay‐Corredera, Carmen, and Jorge Alegre‐Cebollada. “The Mechanics of the Heart: Zooming in on Hypertrophic Cardiomyopathy and CMyBP‐C.” FEBS Letters, vol. 596, no. 6, Mar. 2022, pp. 703–46. DOI.org (Crossref), https://doi.org/10.1002/1873-3468.14301.
Sukumolanan, Pratch, and Soontaree Petchdee. “Prevalence of Cardiac Myosin-Binding Protein C3 Mutations in Maine Coon Cats with Hypertrophic Cardiomyopathy.” Veterinary World, vol. 15, no. 2, Feb. 2022, pp. 502–08. PubMed, https://doi.org/10.14202/vetworld.2022.502-508.
Toepfer, Christopher N., et al. “Hypertrophic Cardiomyopathy Mutations in MYBPC3 Dysregulate Myosin.” Science Translational Medicine, vol. 11, no. 476, Jan. 2019, p. eaat1199. DOI.org (Crossref), https://doi.org/10.1126/scitranslmed.aat1199.
VCV000012415.13 – ClinVar – NCBI. https://www.ncbi.nlm.nih.gov/clinvar/variation/12415/. Accessed 5 Oct. 2022.
VCV000042540.39 – ClinVar – NCBI. https://www.ncbi.nlm.nih.gov/clinvar/variation/42540/?oq=((51710[AlleleID]). Accessed 5 Oct. 2022.
VCV000043627.20 – ClinVar – NCBI. https://www.ncbi.nlm.nih.gov/clinvar/variation/43627/. Accessed 5 Oct. 2022.
VCV000228409.12 – ClinVar – NCBI. https://www.ncbi.nlm.nih.gov/clinvar/variation/228409/. Accessed 5 Oct. 2022.
Zou, Yubao, et al. “Multiple Gene Mutations, Not the Type of Mutation, Are the Modifier of Left Ventricle Hypertrophy in Patients with Hypertrophic Cardiomyopathy.” Molecular Biology Reports, vol. 40, no. 6, June 2013, pp. 3969–76. DOI.org (Crossref), https://doi.org/10.1007/s11033-012-2474-2.

