Key takeaways:
~In small fiber neuropathy, the tiniest nerve fibers break down and cause burning pain, numbness, odd sensations, or autonomic nervous system issues.
~Small fiber neuropathy is a type of peripheral neuropathy, but the symptoms can differ from what you would typically think of as neuropathy.
~ Genetics can play a role in who is more likely to get small fiber neuropathy when triggered by certain factors.
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Join today.
What is Small Fiber Neuropathy?
Nerve cells have long, slender fibers called axons that carry a signal from one nerve to the next.
Nerves are divided into categories of group A, group B, and group C nerve fibers. The group A and B fibers are myelinated, which means that they are insulated with myelin and carry the electrical impulse more quickly to the next neuron. Group C fibers are unmyelinated and carry information that is not needed as quickly, such as warm or cool sensations.
Small fiber neuropathy affects group C neurons and a specific type of myelinated group A neurons called Aδ.
- The type C neurons sense diffuse pain and warmth.
- The Aδ neurons sense pressure, touch, cold, and specific threat signals.
Symptoms of small fiber neuropathy often start with odd sensations in the feet or hands. Some people say ‘pins and needles’; others compare it to ants biting or itchiness. It can progress up the legs and arms and then throughout the body. The abnormal activation of these nerves can cause pain, diffuse sensations, and itching.
Small fiber neuropathy can also cause abnormal activation of autonomic nervous system functions like those in the heart, gastrointestinal tract, or bladder. Fatigue is common in SFN, which is thought to be due to the cardiac issues.[ref]
What goes wrong in small fiber neuropathy?
Small fiber neuropathy (SFN) is described as a structural anomaly of small nerve fibers along with degeneration of the nerve endings. It is diagnosed by a skin biopsy that looks at the tiniest nerve fibers. The biopsy can show fewer than normal small nerve fibers in the skin, which would indicate nerve damage.[ref][ref]
The C-fibers involved in SFN transmit a more dull, diffuse pain sensation, which is a slower nerve conduction reaction. C-fibers are also involved in cold temperature pain (<5oC) and warming skin (41oC).[ref] Damage to the C-fiber neurons can cause temperature sensitivity and diffuse pain.
Sharp, pricking pain in SFN can be due to the involvement of the Aδ-fibers, which also respond to heat. The Aδ-fibers also respond to hair movement (skin hair movement), skin cooling (25oC), heat pain (>45oC), and excess stretching.[ref]
What causes the nerve endings to be damaged or degenerate? SNF can be caused by a variety of factors, according to research.
Causes of SFN can be classified as follows:[ref][ref]
- metabolic diseases (e.g., such as in diabetes)
- toxins (e.g., drugs)
- immune-mediated (e.g., autoimmune disorders, viral infection)
- genetic factors
- B-vitamin deficiency
Small fiber neuropathy and the autonomic nervous system:
It is easy to visualize how the damaged nerves in the skin on your feet or hands could cause pain, burning, numbness, or tingling. However, autonomic nervous system involvement can be harder to envision.
The autonomic nervous system involves all bodily systems that we don’t have to think about — heart rate, digestion, etc. We can divide the autonomic nervous system into categories: the sympathetic system (stress response), the parasympathetic system (rest response), and the enteric nervous system (digestive system).[ref]
Small nerve fibers control the following systems:
- heart rate variability with deep respiration (e.g., Valsalva maneuver)
- heart rate and blood pressure response to postural change (e.g., standing up after lying down)
- urine flow rate and feeling of need to urinate
- isometric exercise
- sweating
- skin wrinkling in water
Thus, damage to the small nerve fibers can cause disruption in heart rate, blood pressure, bladder irritation, lack of sweating, and a lack of skin wrinkling when in water for 30 minutes.[ref][ref]
How do nerves fire?
Nerves send electrical impulses to the brain to activate specific brain functions. Here is an animated image showing how the electrical signal travels along the axon of a nerve cell.
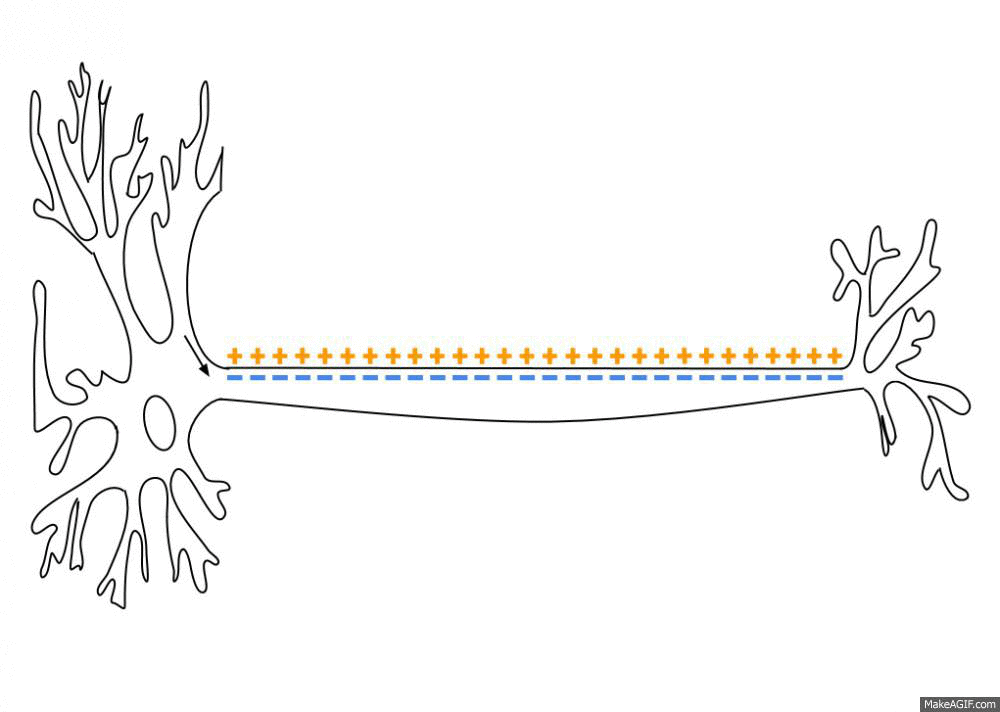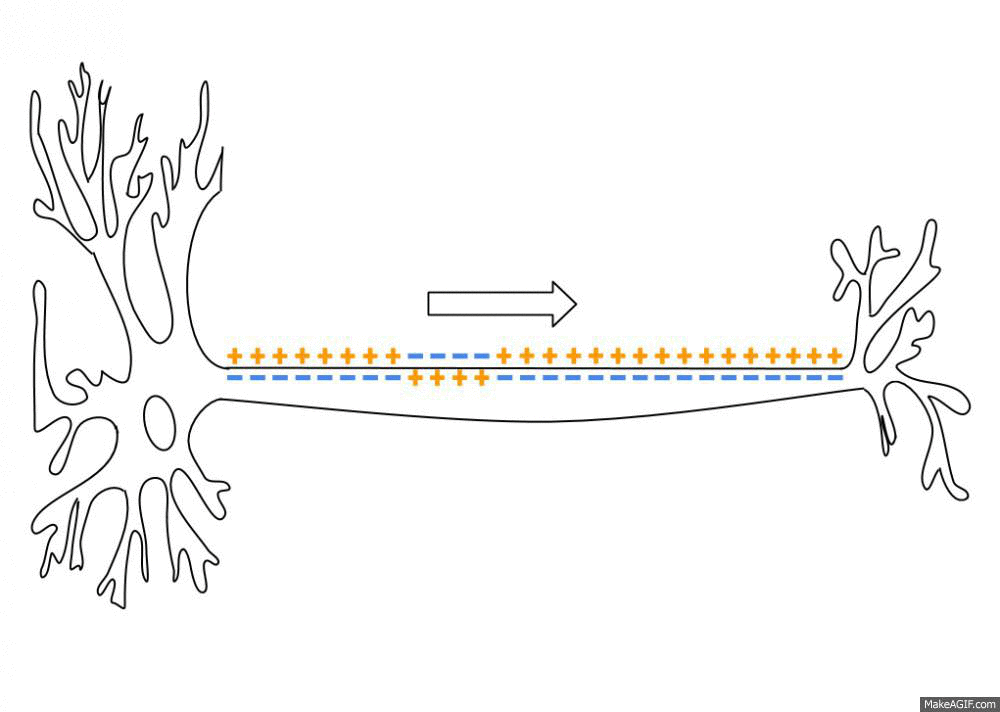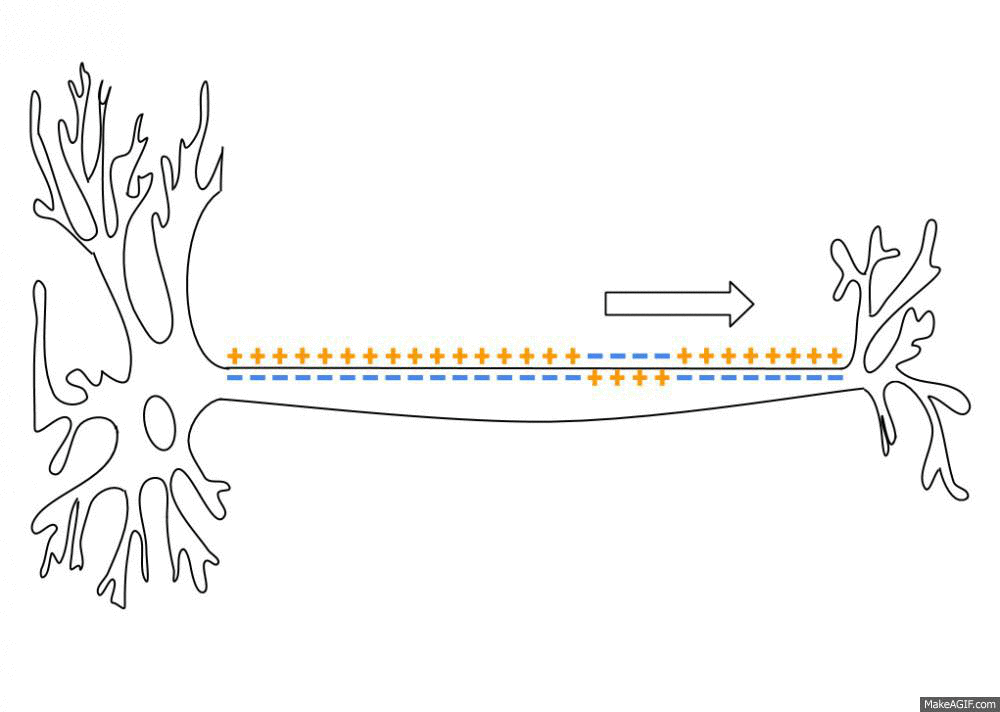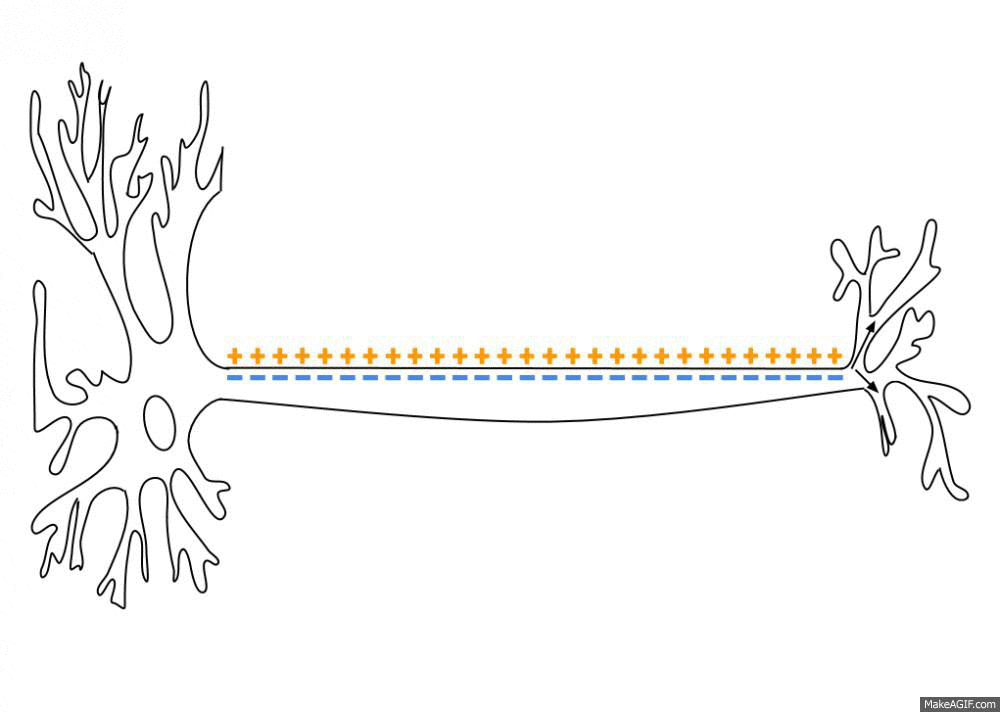
Sodium and potassium ion channels open and close in response to a signal from another neuron (or a receptor). These ion channels allow positive or negative ions in or out of the membrane, thus changing the electrical potential. The buildup of charge then travels along the length of the axon to the axon terminal. The axon terminal can cause the next neuron to fire, relaying the message.
The axon terminal is at the end of the axon, where the signal is relayed to the next neuron. In SFN, this can be damaged or altered. In small fiber neuropathy, biopsy shows reduced nerve endings in the skin, possibly due to the damage. The nerve damage causes pain when there shouldn’t be (mechanical allodynia) and reduced small fiber nerve endings in the skin.[ref]
Genetic research points to a couple of mechanisms:
Genetic mutations in voltage-gated sodium channels are one known cause of small fiber neuropathy. Specifically, mutations in the genes that code for part of the NaV1.7 and NaV1.8 sodium channels have been identified as causing SFN. This type of sodium channel is located in the small nerve fiber cells that transmit pain signals to the brain (nociceptors). These mutations can cause the sodium channel to not completely close when the channel is turned off, or they can cause the sodium channels to open more easily than usual. Either way, increased nerve firing results.[ref]
Another cause of SFN shown by genetics research surrounds the TRPV1 channel. TRPV1 is the transient receptor potential vanilloid subtype 1 receptor, activated by heat and capsaicin (spicy chillis). In neurons, activation of TRPV1 signals for pain — like when you eat a jalapeno. A lack of TRPV1 receptors causes ‘thermal analgesia’ or lack of pain from touching (or eating) hot stuff. While this sounds good, paradoxically, a lack of TRPV1 receptors is associated with SFN.
Research shows that TNF-alpha, an inflammatory cytokine, is involved in neuropathic pain through the activation of TRPV1 channels along with the TNF-alpha receptor.[ref]
Small Fiber Neuropathy can be part of another disorder:
Looking beyond genetics, small fiber neuropathy can be caused by autoimmune disease. Auto-antibodies to neuronal proteins, such as trisulfated heparin disaccharide and fibroblast growth factor, are found in about 20% of patients with SFN.[ref]
Disorders that are associated with small fiber neuropathy include:[ref]
- Hereditary diseases such as Fabry’s disease, Wilson’s disease, familial amyloidosis, and mutations in sodium channels.
- Metabolic diseases such as diabetes or insulin resistance.
- Micronutrient deficiency such as vitamin B12 or copper.
- Infectious diseases such as Lyme, HIV, hepatitis C
- Toxins such as alcohol, chemotherapy, or neurotoxic drugs
- Vaccine reactions
- Autoimmune diseases, including lupus, sarcoidosis, rheumatoid arthritis, chronic inflammatory demyelinating polyneuropathy, lupus, primary systemic amyloidosis, fibromyalgia, celiac, monoclonal gammopathy, and Ehlers-Danlos syndromes
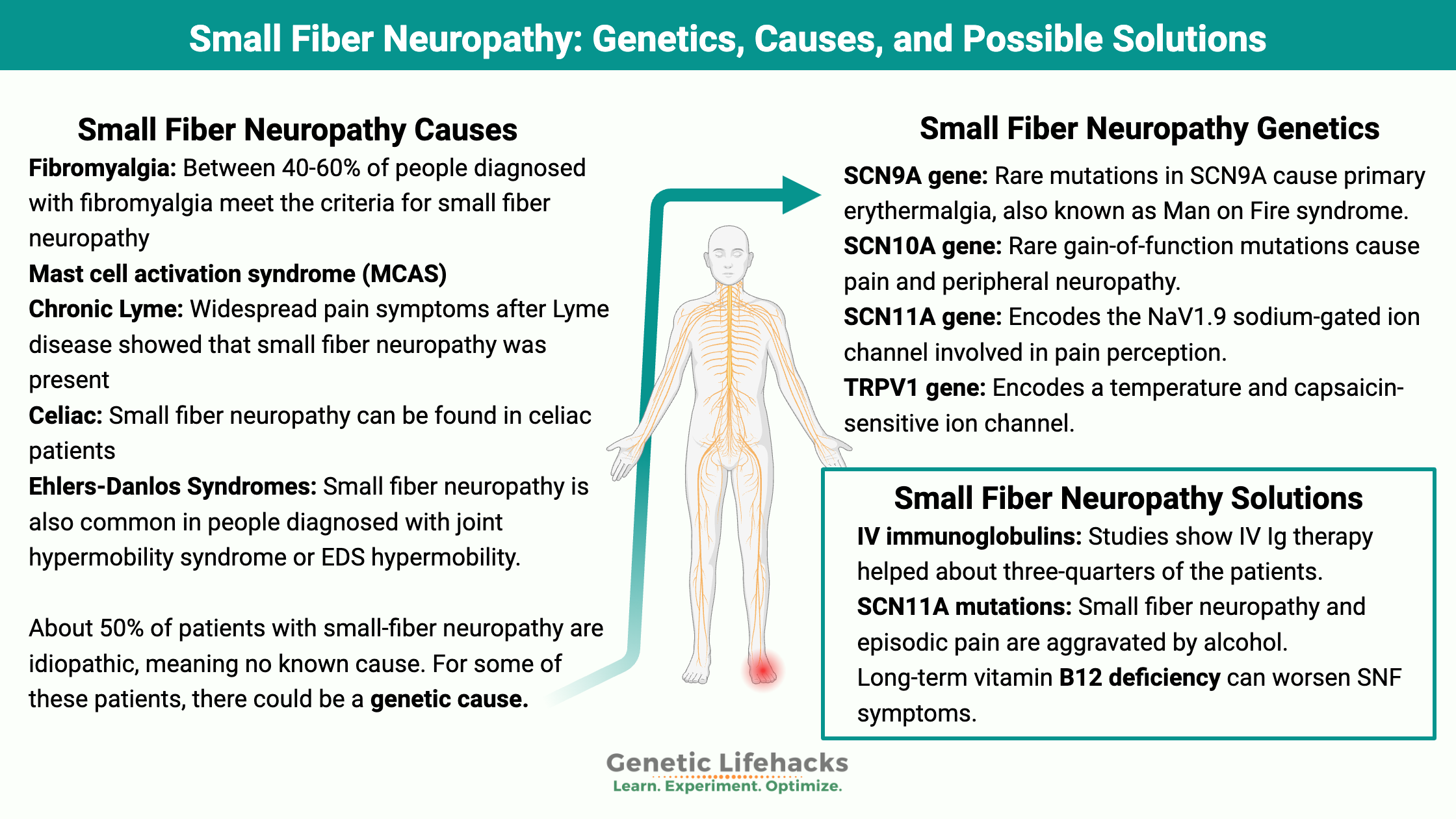
About 50% of patients with small-fiber neuropathy are idiopathic, meaning no known cause.[ref] For some (most?) of these patients, there could be a genetic cause.
Fibromyalgia: Between 40-60% of people diagnosed with fibromyalgia meet the biopsy criteria for small fiber neuropathy, according to one study.[ref]
Mast cell activation syndrome (MCAS) or hereditary alpha tryptasemia (HαT): In a study involving patients with MCAS or HαT, about 80% of each patient group were found to have reduced small nerve fibers consistent with SNF.[ref]
Chronic Lyme: A study in people with widespread pain symptoms after Lyme disease showed that small fiber neuropathy was present in all patients. The patients all responded to IV immunoglobulin therapy.[ref]
Celiac: Small fiber neuropathy was found in celiac patients who also had numbness and tingling in the periphery.[ref]
Ehlers-Danlos Syndromes: Small fiber neuropathy is also common in people diagnosed with joint hypermobility syndrome or EDS hypermobility.[ref]
Small fiber neuropathy and COVID-19:
Several viruses, including HIV, hepatitis C, and now SARS-CoV-2, are associated with an increased risk of small fiber neuropathy.
Studies on SFN in Covid show:
- A study involving 23 patients who had recovered from Covid showed that 91% had changes to the cornea consistent with small fiber neuropathy. Nerves in the cornea express ACE2 receptors as well as other receptors that the SARS-CoV-2 virus can use for entry. Alteration in the corneal nerves can cause sensitivity, pain, and dry eyes.[ref]
- The Mayo Clinic published a study in April of 2021 showing that autonomic dysfunction could follow Covid infections. Among other findings, the study showed that COVID-19 exacerbated small fiber neuropathy in people who already had it.[ref]
- Mt. Sinai also published a study showing small fiber neuropathy developing in patients after COVID-19.[ref]
- Another study from Mass General showed new onset small-fiber neuropathy in patients with long Covid.[ref]
- A case study from September 2020 explained a patient who had previously dealt with Lyme disease. After COVID, the patient developed orthostatic intolerance and small fiber neuropathy, which responded to intravenous immunoglobulin therapy.[ref]
Small Fiber Neuropathy after vaccines:
A rare side effect of several vaccines includes small fiber neuropathy.
The current COVID-19 vaccines are no exception:
- An observational study of 23 people with neuropathic symptoms after a Covid vaccine found that >50% of them met the criteria for new onset SFN (biopsy, symptoms). [ref]
- A case study published in April 2021 outlines the reaction of a 57-year-old woman who developed small fiber neuropathy a week after the Pfizer mRNA vaccine. Antibody testing ruled out a prior covid infection, and she was not on any medications nor an alcoholic. Her symptoms included burning and tingling in the extremities, loss of pinprick, and cold sensations in her feet. The biopsy confirmed the small fiber neuropathy.[ref]
- An investigation published in April 2021 that looked at immune-mediated disease flares after the COVID-19 vaccines showed that flare-ups affected 78% of the patients with autoimmune diseases. This investigation included autoimmune diseases that cause small fiber neuropathy.[ref]
Small Fiber Neuropathy: Genotype Report
Note: 23andMe and AncestryDNA data files do not cover all of the rare mutations in the genes related to hereditary small fiber neuropathy. So you can’t rule anything out with the variants below. Instead, use this information as a way to see which pathways may be impacted by your genes.
Lifehacks:
First some encouragement: I’ve read on some health websites that small-fiber neuropathy is a lifelong, progressive disease. I don’t think this is true, at least not for everyone. Small nerve fibers have the highest regenerative capacity of any nerve. Thus, I want to encourage you to keep looking for solutions and don’t take ‘it’s a lifelong problem’ as a final answer.[ref]
Dietary changes:
If your small fiber neuropathy is related to metabolic health (diabetes, pre-diabetes), then focusing on your diet is an obvious place to start.
- If you don’t know your normal fasting blood glucose levels, you can get a blood glucose meter at most pharmacies or even large grocery stores. They are relatively inexpensive and will give you a baseline to know if you should focus on your blood glucose levels for SFN.
- If your fasting blood glucose is high, talk with your doctor about your option. Dietary interventions can include cutting out sugar and processed foods. Read about the benefits of low-carb diets for diabetes: https://www.virtahealth.com/reversediabetes.
If you think your small fiber neuropathy is due to an autoimmune disease, look into the Autoimmune Paleo (AIP) diet. It essentially cuts out processed foods and common allergens. The diet focuses on nutrient-dense whole foods.
Testing to confirm:
Talk with your doctor or neurologist about getting tested for small fiber neuropathy. Testing via skin biopsy is available, and your doctor can order the test kits and do it right in the clinic.
IV immunoglobulins:
A 2018 study showed that IVIg therapy helped about three-quarters of the patients with small fiber neuropathy associated with an autoimmune disease.[ref] Talk with your doctor about whether IV immunoglobulin therapy would be a good option for your situation. IVIg therapy isn’t without possible side effects, and it is costly. Be sure to investigate thoroughly and have an educated conversation with your doctor about how to mitigate side effects and how to navigate insurance reimbursement.
Prescription medications:
Your doctor may also have recommendations for prescription medications. In a small clinical trial, both gabapentin and tramadol were more effective than diphenhydramine for SFN pain. Both helped about 1 out of 4 study participants with the pain.[ref] Do some research on the significant side effects of both of those medications before you talk with your doctor about them so that you can make an educated decision.
SCN9A gain of function mutations:
Related Articles and Topics:
References:
Abrams, Rory M. C., et al. “Small Fiber Neuropathy Associated with SARS-CoV-2 Infection.” Muscle & Nerve, vol. 65, no. 4, Apr. 2022, pp. 440–43. PubMed, https://doi.org/10.1002/mus.27458.
Barros, Alberto, et al. “Small Fiber Neuropathy in the Cornea of Covid-19 Patients Associated with the Generation of Ocular Surface Disease.” The Ocular Surface, vol. 23, Jan. 2022, pp. 40–48. PubMed Central, https://doi.org/10.1016/j.jtos.2021.10.010.
Basantsova, Natalia Y., et al. “Small-Fiber Neuropathy Definition, Diagnosis, and Treatment.” Neurological Sciences, vol. 40, no. 7, July 2019, pp. 1343–50. Springer Link, https://doi.org/10.1007/s10072-019-03871-x.
Bautista, J., et al. “Regenerative Plasticity of Intact Human Skin Axons.” Journal of the Neurological Sciences, vol. 417, Oct. 2020, p. 117058. PubMed, https://doi.org/10.1016/j.jns.2020.117058.
Binder, Andreas, et al. “Transient Receptor Potential Channel Polymorphisms Are Associated with the Somatosensory Function in Neuropathic Pain Patients.” PloS One, vol. 6, no. 3, Mar. 2011, p. e17387. PubMed, https://doi.org/10.1371/journal.pone.0017387.
Brannagan, Thomas H., III, et al. “Small-Fiber Neuropathy/Neuronopathy Associated With Celiac Disease: Skin Biopsy Findings.” Archives of Neurology, vol. 62, no. 10, Oct. 2005, pp. 1574–78. Silverchair, https://doi.org/10.1001/archneur.62.10.1574.
Cazzato, Daniele, et al. “Small Fiber Neuropathy Is a Common Feature of Ehlers-Danlos Syndromes.” Neurology, vol. 87, no. 2, July 2016, pp. 155–59. PubMed Central, https://doi.org/10.1212/WNL.0000000000002847.
Chang, Chin-Hong, et al. “Transient Receptor Potential Vanilloid Subtype 1 Depletion Mediates Mechanical Allodynia through Cellular Signal Alterations in Small-Fiber Neuropathy.” Pain Reports, vol. 6, no. 1, Apr. 2021, p. e922. PubMed Central, https://doi.org/10.1097/PR9.0000000000000922.
Chávez-Castillo, Mervin, et al. “Specialized Pro-Resolving Lipid Mediators: The Future of Chronic Pain Therapy?” International Journal of Molecular Sciences, vol. 22, no. 19, Sept. 2021, p. 10370. PubMed Central, https://doi.org/10.3390/ijms221910370.
de Greef, B. T. A., et al. “Associated Conditions in Small Fiber Neuropathy – a Large Cohort Study and Review of the Literature.” European Journal of Neurology, vol. 25, no. 2, Feb. 2018, pp. 348–55. PubMed Central, https://doi.org/10.1111/ene.13508.
Duan, Guangyou, et al. “A SCN10A SNP Biases Human Pain Sensitivity.” Molecular Pain, vol. 12, Sept. 2016, p. 1744806916666083. PubMed Central, https://doi.org/10.1177/1744806916666083.
Egenolf, Nadine, et al. “Diagnosing Small Fiber Neuropathy in Clinical Practice: A Deep Phenotyping Study.” Therapeutic Advances in Neurological Disorders, vol. 14, Mar. 2021, p. 17562864211004318. PubMed Central, https://doi.org/10.1177/17562864211004318.
Faber, Catharina G., et al. “Gain of Function Naν1.7 Mutations in Idiopathic Small Fiber Neuropathy.” Annals of Neurology, vol. 71, no. 1, Jan. 2012, pp. 26–39. PubMed, https://doi.org/10.1002/ana.22485.
Farhad, Khosro. “Current Diagnosis and Treatment of Painful Small Fiber Neuropathy.” Current Neurology and Neuroscience Reports, vol. 19, no. 12, Nov. 2019, p. 103. PubMed, https://doi.org/10.1007/s11910-019-1020-1.
Gonzalez-Lopez, Eugene, et al. “Homozygosity for the SCN10A Polymorphism Rs6795970 Is Associated With Hypoalgesic Inflammatory Bowel Disease Phenotype.” Frontiers in Medicine, vol. 5, Nov. 2018, p. 324. PubMed Central, https://doi.org/10.3389/fmed.2018.00324.
Han, Chongyang, et al. “The G1662S NaV1.8 Mutation in Small Fibre Neuropathy: Impaired Inactivation Underlying DRG Neuron Hyperexcitability.” Journal of Neurology, Neurosurgery, and Psychiatry, vol. 85, no. 5, May 2014, pp. 499–505. PubMed, https://doi.org/10.1136/jnnp-2013-306095.
Hemminger, Adam, and Brandon K. Wills. “Vitamin B6 Toxicity.” StatPearls, StatPearls Publishing, 2022. PubMed, http://www.ncbi.nlm.nih.gov/books/NBK554500/.
Hovaguimian, Alexandra, and Christopher H. Gibbons. “Diagnosis and Treatment of Pain in Small Fiber Neuropathy.” Current Pain and Headache Reports, vol. 15, no. 3, June 2011, pp. 193–200. PubMed Central, https://doi.org/10.1007/s11916-011-0181-7.
Kelley, Mary A., and Kevin V. Hackshaw. “Intraepidermal Nerve Fiber Density as Measured by Skin Punch Biopsy as a Marker for Small Fiber Neuropathy: Application in Patients with Fibromyalgia.” Diagnostics, vol. 11, no. 3, Mar. 2021, p. 536. PubMed Central, https://doi.org/10.3390/diagnostics11030536.
Leuti, Alessandro, et al. “Role of Specialized Pro-Resolving Mediators in Neuropathic Pain.” Frontiers in Pharmacology, vol. 12, Aug. 2021, p. 717993. PubMed Central, https://doi.org/10.3389/fphar.2021.717993.
Li, Qingqin S., et al. “SCN9A Variants May Be Implicated in Neuropathic Pain Associated With Diabetic Peripheral Neuropathy and Pain Severity.” The Clinical Journal of Pain, vol. 31, no. 11, Nov. 2015, pp. 976–82. PubMed Central, https://doi.org/10.1097/AJP.0000000000000205.
Liu, Baowen, et al. “The Role of Voltage-Gated Sodium Channel 1.8 in the Effect of Atropine on Heart Rate: Evidence From a Retrospective Clinical Study and Mouse Model.” Frontiers in Pharmacology, vol. 11, July 2020, p. 1163. PubMed Central, https://doi.org/10.3389/fphar.2020.01163.
Liu, Xiaolei, et al. “IVIg for Apparently Autoimmune Small-Fiber Polyneuropathy: First Analysis of Efficacy and Safety.” Therapeutic Advances in Neurological Disorders, vol. 11, Jan. 2018, p. 1756285617744484. PubMed Central, https://doi.org/10.1177/1756285617744484.
Long, Wentong, et al. “TRPV1 Channels as a Newly Identified Target for Vitamin D.” Channels, vol. 15, no. 1, pp. 360–74. PubMed Central, https://doi.org/10.1080/19336950.2021.1905248. Accessed 12 May 2022.
Matthews, Catherine A., et al. “Small Fiber Polyneuropathy as a Potential Therapeutic Target in Interstitial Cystitis/Bladder Pain Syndrome.” International Urogynecology Journal, vol. 30, no. 11, Nov. 2019, pp. 1817–20. PubMed Central, https://doi.org/10.1007/s00192-019-04011-x.
Neff, Robert A., and Alan D. Wickenden. “Selective Targeting of Nav1.7 with Engineered Spider Venom-Based Peptides.” Channels, vol. 15, no. 1, pp. 179–93. PubMed Central, https://doi.org/10.1080/19336950.2020.1860382. Accessed 12 May 2022.
NM_002977.3(SCN9A):C.721T>A (p.Ser241Thr) AND Primary Erythromelalgia – ClinVar – NCBI. https://www.ncbi.nlm.nih.gov/clinvar/RCV000006723.3/. Accessed 12 May 2022.
Novak, Peter, Donna Felsenstein, et al. “Association of Small Fiber Neuropathy and Post Treatment Lyme Disease Syndrome.” PLOS ONE, vol. 14, no. 2, Feb. 2019, p. e0212222. PLoS Journals, https://doi.org/10.1371/journal.pone.0212222.
Novak, Peter, Matthew P. Giannetti, et al. “Mast Cell Disorders Are Associated with Decreased Cerebral Blood Flow and Small Fiber Neuropathy.” Annals of Allergy, Asthma & Immunology, vol. 128, no. 3, Mar. 2022, pp. 299-306.e1. www.annallergy.org, https://doi.org/10.1016/j.anai.2021.10.006.
Novak, Peter. “Post COVID-19 Syndrome Associated with Orthostatic Cerebral Hypoperfusion Syndrome, Small Fiber Neuropathy and Benefit of Immunotherapy: A Case Report.” ENeurologicalSci, vol. 21, Dec. 2020, p. 100276. PubMed, https://doi.org/10.1016/j.ensci.2020.100276.
Oaklander, Anne Louise, et al. “Peripheral Neuropathy Evaluations of Patients With Prolonged Long COVID.” Neurology(R) Neuroimmunology & Neuroinflammation, vol. 9, no. 3, May 2022, p. e1146. PubMed, https://doi.org/10.1212/NXI.0000000000001146.
Okuda, Hiroko, et al. “Infantile Pain Episodes Associated with Novel Nav1.9 Mutations in Familial Episodic Pain Syndrome in Japanese Families.” PLoS ONE, vol. 11, no. 5, May 2016, p. e0154827. PubMed Central, https://doi.org/10.1371/journal.pone.0154827.
Palugulla, Sreenivasulu, et al. “Association of Voltage-Gated Sodium Channel Genetic Polymorphisms with Oxaliplatin-Induced Chronic Peripheral Neuropathy in South Indian Cancer Patients.” Asian Pacific Journal of Cancer Prevention : APJCP, vol. 18, no. 11, 2017, pp. 3157–65. PubMed Central, https://doi.org/10.22034/APJCP.2017.18.11.3157.
Price, Nicola, et al. “Safety and Efficacy of a Topical Sodium Channel Inhibitor (TV-45070) in Patients With Postherpetic Neuralgia (PHN).” The Clinical Journal of Pain, vol. 33, no. 4, Apr. 2017, pp. 310–18. PubMed Central, https://doi.org/10.1097/AJP.0000000000000408.
Raasing, Lisette R. M., et al. “Current View of Diagnosing Small Fiber Neuropathy.” Journal of Neuromuscular Diseases, vol. 8, no. 2, pp. 185–207. PubMed Central, https://doi.org/10.3233/JND-200490. Accessed 12 May 2022.
—. “Current View of Diagnosing Small Fiber Neuropathy.” Journal of Neuromuscular Diseases, vol. 8, no. 2, pp. 185–207. PubMed Central, https://doi.org/10.3233/JND-200490. Accessed 12 May 2022.
Rs483352921 RefSNP Report – DbSNP – NCBI. https://www.ncbi.nlm.nih.gov/snp/rs483352921#clinical_significance. Accessed 12 May 2022.
Serhan, Charles N., et al. “E-Series Resolvin Metabolome, Biosynthesis and Critical Role of Stereochemistry of Specialized pro-Resolving Mediators (SPMs) in Inflammation-Resolution: Preparing SPMs for Long COVID-19, Human Clinical Trials, and Targeted Precision Nutrition.” Seminars in Immunology, Feb. 2022, p. 101597. PubMed Central, https://doi.org/10.1016/j.smim.2022.101597.
Shouman, Kamal, et al. “Autonomic Dysfunction Following COVID-19 Infection: An Early Experience.” Clinical Autonomic Research: Official Journal of the Clinical Autonomic Research Society, vol. 31, no. 3, June 2021, pp. 385–94. PubMed, https://doi.org/10.1007/s10286-021-00803-8.
Stracke, H., et al. “Benfotiamine in Diabetic Polyneuropathy (BENDIP): Results of a Randomised, Double Blind, Placebo-Controlled Clinical Study.” Experimental and Clinical Endocrinology & Diabetes: Official Journal, German Society of Endocrinology [and] German Diabetes Association, vol. 116, no. 10, Nov. 2008, pp. 600–05. PubMed, https://doi.org/10.1055/s-2008-1065351.
Valdes, Ana M., et al. “The Ile585Val TRPV1 Variant Is Involved in Risk of Painful Knee Osteoarthritis.” Annals of the Rheumatic Diseases, vol. 70, no. 9, Sept. 2011, pp. 1556–61. PubMed, https://doi.org/10.1136/ard.2010.148122.
VCV000069850.3 – ClinVar – NCBI. https://www.ncbi.nlm.nih.gov/clinvar/variation/69850/?oq=((80741[AlleleID]))&m=NM_001349253.2(SCN11A):c.673C%3ET%20(p.Arg225Cys). Accessed 12 May 2022.
Wadhawan, Samir, et al. “NaV Channel Variants in Patients with Painful and Nonpainful Peripheral Neuropathy.” Neurology. Genetics, vol. 3, no. 6, Dec. 2017, p. e207. PubMed, https://doi.org/10.1212/NXG.0000000000000207.
Waheed, Waqar, et al. “Post COVID‐19 Vaccine Small Fiber Neuropathy.” Muscle & Nerve, vol. 64, no. 1, July 2021, pp. E1–2. PubMed Central, https://doi.org/10.1002/mus.27251.
Watad, Abdulla, et al. “Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following MRNA/DNA SARS-CoV-2 Vaccination.” Vaccines, vol. 9, no. 5, Apr. 2021, p. 435. PubMed, https://doi.org/10.3390/vaccines9050435.
Woods, Christopher Geoffrey, et al. “The Phenotype of Congenital Insensitivity to Pain Due to the NaV1.9 Variant p.L811P.” European Journal of Human Genetics, vol. 23, no. 5, May 2015, pp. 561–63. PubMed Central, https://doi.org/10.1038/ejhg.2014.166.
Yang, Luyao, et al. “Alcohol-Aggravated Episodic Pain in Humans with SCN11A Mutation and ALDH2 Polymorphism.” Pain, vol. 161, no. 7, July 2020, pp. 1470–82. PubMed, https://doi.org/10.1097/j.pain.0000000000001853.