This article is a departure from my usual writings on how genetic variants interact with foods or health issues. Instead, I’m going to pull together recent research studies in an effort to puzzle out a cause of some of the constellation of issues surrounding long Covid and/or post-vaccine.
Let me set the stage: The stories that I’ve heard from friends, Genetic Lifehacks members, and via many online anecdotes often go like this:
After covid and/or after the mRNA vaccines, the individual starts having heart palpitations and a racing heart. Blood pressure shoots up periodically. Their heart rhythm issues are described as palpitations, PVCs, skipped beats, or a-fib.
The racing heart rate, or tachycardia, seems to happen randomly for some people – while driving, sitting around at home, or when exercising. For others, the tachycardia just happens when they stand up.
Accompanying these heart rhythm issues are a range of symptoms, including dizziness, tinnitus (ringing in the ears), chest pains, fatigue, muscle twitches, and just not feeling right. Some people end up in the ER or urgent care; others head to the cardiologist to get their heart thoroughly checked out with CT scans, stress tests, and holter monitors.
These anecdotal stories often wind up with… The cardiologist says nothing is really wrong, and now I have a prescription for a blood pressure medicine. It’s good that there isn’t heart damage, but it is frustrating for both the doctors and patients to have an unknown cause.
We take the rhythm of our hearts for granted. We expect it to carry on in a regular fashion, not too fast and not too slow. Although some people have naturally higher or lower heart rates and blood pressure, most of us who are still kicking today have a heart rhythm that stays in a normal range.
Several research papers have studied the link between long Covid and heart rhythm disorders. In December 2021, a meta-analysis showed that about 20% of people with long Covid continued to have heart palpitations.[ref] Statistics are harder to come by for heart palpitations or tachycardia after the mRNA vaccines. Case reports highlight several individual cases, but I am not finding longitudinal studies.[ref][ref][ref]
Related article: Long Spike
Histamine, Heart Rhythm, Spike Protein, Mast Cells – oh my!
I’m going to explore the research linking histamine, mast cells, and the spike protein.
Let’s start with an explanation of histamine and its roles in the body.
Histamine is a biogenic amine produced by your cells, by the gut microbiome, and also found in foods. It is produced by converting the amino acid histidine into histamine through a decarboxylation reaction. Other biogenic amines include tyramine, cadaverine, and putrescine. Biogenic amines are required in just the right amount and in the right tissues. High levels of biogenic amines can cause negative effects (diarrhea, food poisoning, vomiting, sweating, or tachycardia) if consumed or produced in excess .[ref]
Histamine is most commonly associated with allergic reactions, such as when your nose runs due to pollen allergies in the spring. But there is much more to the histamine story than just watery eyes and a runny nose during allergy season.
Histamine is a signaling molecule that causes an action to take place depending on which receptor it binds to.
For more than a century, it has been known that histamine causes the heart rate to increase and heart contractions to be more forceful. Histamine was initially linked to heart arrhythmias, according to early research.
How can one molecule, histamine, cause such different actions?
Four different histamine receptors cause histamine actions:[ref]
H1 receptors:
These receptors are found in smooth muscle, endothelial cells (lining the blood vessels), central nervous system, cardiac muscle, and mast cells. Activating the H1 receptors causes allergy-type symptoms such as itching, swelling, vasodilation, nose running, and skin reactions.
H2 receptors:
When histamine activates the H2 receptors in the stomach, acid is released. In the heart, H2 receptors are important in controlling the rhythm. H2 receptors are also found in the intestinal tract, cardiac muscles, and the walls of blood vessels. Mast cells also have H2 receptors, which, when activated, cause more histamine release.
H3 receptors:
The central and peripheral nervous systems contain H3 receptors, which act as a feedback loop for histamine levels in the brain. Activating the H3 receptors impacts serotonin, norepinephrine, and acetylcholine release.[ref]
H4 receptors:
These histamine receptors are at the core of the inflammatory response. H4 receptors are found in the bone marrow, basophils (a type of white blood cell), the thymus, small intestine, spleen, colon, and mast cells.[ref]
H2 receptors and medications:
In the early 1990s, an H2 receptor agonist (a drug that binds to the H2 receptor and mimics histamine) was tested in patients with heart failure. The medication would cause the heart to beat more forcefully, which can help in heart failure.
Unfortunately, the clinical trials showed that long-term use of that specific H2 receptor agonist increased the risk of cardiac death, hypotension, and syncope.[ref] It is currently used only as an hospital IV medication for short periods of time.
The H2 receptor is also found in the stomach, where it regulates the amount of stomach acid released when you eat.
In the 1970s and 80s, H2-blocker medications such as famotidine, ranitidine, and cimetidine became popular as a way of stopping acid reflux. These drugs inhibit the H2 receptor, preventing histamine from binding to it and activating it. This inhibition reduces the amount of gastric acid produced in the stomach.
Histamine in regulating the heartbeat:
The sinus node is the heart’s natural pacemaker. This pacemaker sends out electrical impulses that spread out like a wave, forcing the top chamber to contract before the bottom chamber contracts.
Before going further, I want to clarify that histamine is only part of the picture in heart rate regulation. Your heartbeat has many redundancies and multiple pathways involved in regulation.[ref – open source, good overview]
All four histamine receptors are found in the heart, but the H2 and H1 receptors are the main receptors involved in contraction and heart rate.[ref]
Histamine binding to the H2 receptor impacts both the rhythm of the heart and the force with which it beats.
Animal studies show that H2-receptor blockers can stop heart arrhythmia. However, increasing the amount of histamine and synthetic H2 receptor agonists causes more arrhythmias. Additionally, researchers created a transgenic model with more H2 receptors in the cardiac muscle cells, leading to an increase in heart arrhythmia.[ref]
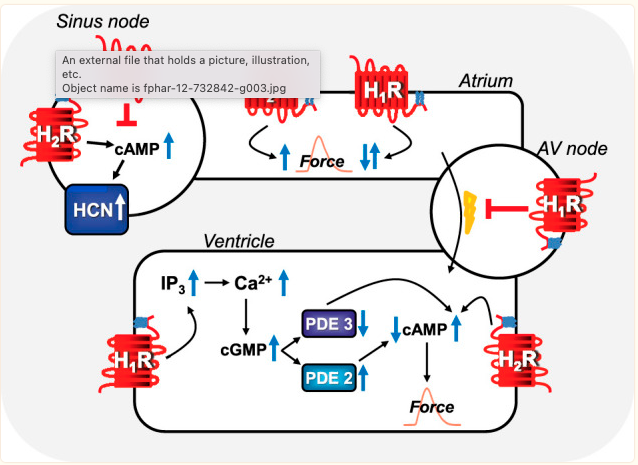
Histamine has been linked to heart arrhythmias since the 1920s.
Injecting histamine can produce tachycardia (high heart rate), according to another study from the 1930s. Animal studies show that histamine and histamine receptors are intricately involved in heart rhythm. Interestingly, animal studies of arrhythmia in septic shock showed that a combination of H1 and H2 receptor blockers is effective.[ref]
The animal results seem to hold true in humans, too. According to a study published as a letter to the editor in the International Journal of Cardiology, patients with atrial fibrillation had significantly greater histamine levels on average. The study group consisted of 47 patients with acute atrial fibrillation, compared to 20 healthy controls.[ref]
Additionally, and perhaps more convincingly, patients with mastocytosis have an increased incidence of arrhythmias. Mastocytosis increases the number of mast cells, which give off large amounts of histamine when activated.[ref]
H2 receptor antagonists (such as for heartburn) are also linked to decreased mortality in patients with heart failure. The study involved over 10,000 heart failure patients and showed a ~25% reduction in mortality at 30 days. [ref]
Histamine in Heart Remodeling:
Before going further into the adverse effects of too much histamine, I want to take a minute to point out that histamine plays multiple roles in heart health. It is easy to get into the ‘histamine is bad‘ mindset, but we need to keep in mind that histamine is an essential signaling molecule that serves various functions in the body.
I mentioned above that cardiac muscles have both H2 and H1 receptors. Recent animal studies show that histamine plays a positive role in heart remodeling after a heart attack. After a heart attack, there is increased cell death and fibrosis if there isn’t enough histamine. Thus, histamine plays a positive role in the remodeling of cardiac muscle cells following myocardial infarction. [ref][ref]
Another animal study discovered that histamine is required for heart remodeling. H1 receptors and histamine were discovered to be critical in cardiac stem cells being triggered to restore damaged heart tissue, according to the study.[ref]
Histamine formation and metabolism:
As mentioned above, histamine creation can occur in cells via a reaction that transforms the amino acid histidine into histamine.
Histamine can also be consumed via foods, and it can be created by certain types of bacteria in the gut.
- Foods high in histamine include fermented foods (wine, aged cheeses), spinach, tomatoes, chocolate, strawberries, deli meats, seafood that isn’t completely fresh — and more. Read through my article on histamine intolerance for more details.
- Allergic reactions cause the release of a lot of histamine from mast cells.
- In the brain, neurons produce histamine, which is used as a neurotransmitter. Histamine as a neurotransmitter is involved in appetite, body temperature, and wakefulness.
Histamine is broken down in the intestines by an enzyme called diamine oxidase (DAO), which is produced in the intestines, kidneys, and placenta. In tissues throughout the body, the enzyme histamine n-methyltransferase (HNMT) metabolizes (breaks down) histamine by adding a methyl group to it.
What does all of this have to do with the SARS-CoV-2 spike protein?
A recent study showed that the spike protein could bind to mast cells and cause degranulation. The spike protein is part of the SARS-CoV-2 virus and the mRNA vaccines.[ref]

Another study shows that histamine potentiates the interaction with the spike protein and the ACE2 receptor in epithelial cells (cells that line the blood vessels).[ref]
Both of these studies are interesting in that they tie together the spike protein, histamine, and mast cells to the constellation of symptoms people report (heart arrhythmia, tachycardia, blood pressure regulation issues, dizziness, fatigue, etc.).
Another study showed that the spike protein induces mast cell degranulation, and that antihistamines could reduce lung damage. The study was done in mice.[ref]
Let’s dig into the mast cell aspect for a bit…
Mast Cells: Immune response to SARS-CoV-2 and Spike Protein
Mast cells are immune system cells found in all tissues of the body. They are usually found in close proximity to blood vessels, skin, the intestinal tract, lungs, and other areas exposed to outside stimuli. They originate from stem cells in the bone marrow.[ref]
Mast cells are part of our immune response, and they react to allergens, pathogens, and other proteins by bursting open and spilling out inflammatory molecules, including histamine, tryptase, serotonin, inflammatory lipids, and inflammatory cytokines.
Mast cells have a bunch of different receptors on their cell membranes. It means that they can be activated by a range of different things — from allergens to viruses to IgE antibodies to damaged parts of a cell.
The ACE2 receptor is found on the cell membrane of many cell types, including mast cells. Thus, the SARS-CoV-2 spike protein can activate mast cells and trigger degranulation.[ref]
In the lungs, mast cell degranulation is thought to cause the lung injury seen in COVID-19. Researchers have found that mast cell stabilizer drugs that block degranulation can dampen the SARS-CoV-2 inflammatory response in the lungs, which causes lung injury.[ref]
Mast cell response to the SARS-CoV-2 spike protein isn’t just limited to lung cells. Other research studies show that there is rapid mast cell degranulation in multiple tissues when exposed to either the full SARS-CoV-2 virus or just the spike protein.[ref]
In a feedback regulation loop that may enhance the inflammatory response, histamine is not only released by mast cells but can also cause mast cell degranulation.[ref]
Put this together with the new research studies showing that histamine potentiates the interaction with the spike protein as well as the research showing the spike protein binds to mast cells…[ref][ref] The result could be a continuing activation of mast cells and histamine release, which impacts heart rate, heart rhythm, etc.
I keep talking about the spike protein, and let me explain why I am explicitly referring to it this way.
The SARS-CoV-2 virus uses the spike protein to attach to and enter host cells, which is the obvious interaction here. But researchers now find that the spike protein is found in exosomes in the plasma of patients with mild or severe COVID-19. Exosomes are small vesicles released from cells during lots of different conditions (normal). They shuttle proteins, lipids, and RNA between cells. Important here is that the spike protein is being found in exosomes in the plasma of Covid patients.[ref]
In April of 2021, researchers from the Salk Institute published a study showing that the spike protein alone could damage endothelial cells (the cells that line your blood vessels).[ref]
The spike protein from SARS-CoV-2 was also found in the monocytes of patients with long Covid up to 15 months after infection.[ref]
The spike protein is also produced in the body from the mRNA in covid vaccines. While the initial assumption was that these spike proteins would stay in the arm where they were injected, that didn’t turn out to be the case. A recent study published in Cell showed that the mRNA and spike antigen were found in lymph nodes eight weeks after vaccination in some people.[ref]
Mast cell activation syndrome:
Before going further, let me explain what happens when your mast cells are activated too easily and on an ongoing basis:
Mast Cell Activation Syndrome (MCAS) is a continual hypersensitivity to environmental triggers (foods, chemicals, smells, temperature), causing mast cells to degranulate more easily than they should. Symptoms of MCAS include itching, flushing, abdominal issues, brain fog, lightheadedness, nasal congestion, and cardiovascular issues such as tachycardia and hypotensive syncope.[ref]
Related article: Mast Cell Activation Syndrome
Autonomic dysfunction?
At this point in the article, you may be wondering: Is everything related to long covid or post-vaccine issues only due to histamine and mast cells?
I don’t want to leave you with that impression. The research on both topics points to multiple possible causes and intricate interactions.
In fact, a recent survey of over 2,300 long covid patients found that over 2/3 of the respondents had symptoms that suggested moderate to severe autonomic dysfunction.[ref] (Which leaves 1/3 of respondents without autonomic dysfunction…)
The autonomic nervous system controls body systems that we don’t have to think about — digestion, heart rate, breathing, urination, and arousal.
While heart rate is a part of the autonomic nervous system, there can be much more to autonomic dysfunction, or dysautonomia, than just issues with heart rate, blood pressure, and arrhythmia.
Mast cell activation may go hand-in-hand with dysautonomia for some people, though. For example, mast cell degranulation is a part of post-exercise hypotension (blood pressure tanking after exercise).[ref][ref] A study in the Journal of the American Heart Association showed that 44% of patients with POTS, a form of dysautonomia, also met the criteria for mast cell activation disorder.[ref]
Related article: POTS and genetics
Polyethylene Glycol – Allergic reactions?
One possibility for mast cell activation after a vaccine is an allergic reaction, which causes mast cells to degranulate. In addition to the spike protein, the mRNA vaccines also contain lipid nanoparticles and other components that are a rare cause of reactions.
One of the components in mRNA vaccines is polyethylene glycol (PEG), which is used to stabilize the lipid nanoparticles. PEG is a known allergen for some people. PEG comes in multiple molecular weights, so people who are allergic to PEG in some medications may not be allergic to it in all forms.[ref][ref]
An allergy to PEG can cause mast cells to degranulate and release histamine. Some research points to prophylactically using mast cell stabilizers to prevent allergic reactions to the covid mRNA vaccines.[ref] Keep in mind that allergic reactions to PEG should happen pretty quickly after the jab. I cannot find research showing how long PEG stays in the system after vaccination.
Other symptoms, such as nerve issues and tinnitus:
In addition to heart-related autonomic issues, some people report problems with nerve activation (zinging, twitching) and tinnitus (ears ringing).
Mast cell activation is one possible reason for both of these.
Similar to the cardiac symptoms, I want to make it clear that mast cell activation is just one possible cause of both nerve activation and tinnitus.
A recent study (March 2022) looked at small fiber neuropathy in people with mast cell activation syndrome. The biopsy results showed that the majority (over 80%) of the MCAS patients had reductions in small nerves similar to what is seen in small fiber neuropathy.[ref]
Small fiber neuropathy can cause pain or nerve activation symptoms in the nerves of the feet, hands, and legs or a more generalized whole body pain. It can be a sudden shooting pain or pain/irritation from something that normally wouldn’t cause pain. Instead of a pain that you would expect from something sharp poking the skin, people with small fiber neuropathy will have irritation and pain from light pressure that shouldn’t cause pain.[ref][ref]
Related article: Small fiber neuropathy, vaccines, and genetics
For some people, tinnitus also is linked to high histamine levels. One of the symptoms of mast cell activation syndrome is tinnitus.[ref] Additionally, H4 histamine receptor antagonists are a possible drug solution for tinnitus.[ref]
Additionally, reports and case studies show that tinnitus can occur after mRNA and other vaccines.[ref]
Related article: Tinnitus and Meniere’s Disease
Lifehacks: Blocking histamine and mast cell degranulation
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Join today.
Related Articles and Topics:
Recipes and Foods for Histamine Intolerance
Interested in low histamine foods and recipes? This article focuses on foods high in histamine so you can easily eliminate them from your diet.
Inflammation: Causes and Natural Solutions
Take a deep dive into the causes of chronic inflammation and learn how to target specific inflammatory pathways to reverse or prevent chronic disease.
Specialized Pro-resolving Mediators: Getting Rid of Chronic Inflammation
Chronic inflammation is at the root of all diseases. New research discusses how pro-resolving mediators are the key to the resolution of inflammation.
C-Reactive Protein Gene: Marker of Inflammation
Inflammation is the driver of many common diseases such as heart attacks, diabetes, obesity, and autoimmune diseases. C-Reactive Protein is a marker of inflammation. Genetic variants can increase or decrease CRP levels. Check your genetic raw data for risk.
References:
Afrin, Lawrence B., et al. “Covid-19 Hyperinflammation and Post-Covid-19 Illness May Be Rooted in Mast Cell Activation Syndrome.” International Journal of Infectious Diseases, vol. 100, Nov. 2020, pp. 327–32. ScienceDirect, https://doi.org/10.1016/j.ijid.2020.09.016.
Alm, P. E. “Sodium Fluoride Evoked Histamine Release from Mast Cells. A Study of Cyclic AMP Levels and Effects of Catecholamines.” Agents and Actions, vol. 13, no. 2–3, Apr. 1983, pp. 132–37. PubMed, https://doi.org/10.1007/BF01967316.
Bigini, P., et al. “The Role and Impact of Polyethylene Glycol on Anaphylactic Reactions to COVID-19 Nano-Vaccines.” Nature Nanotechnology, vol. 16, no. 11, Nov. 2021, pp. 1169–71. www.nature.com, https://doi.org/10.1038/s41565-021-01001-3.
Che, Denis Nchang, et al. “Fisetin Inhibits IL-31 Production in Stimulated Human Mast Cells: Possibilities of Fisetin Being Exploited to Treat Histamine-Independent Pruritus.” Life Sciences, vol. 201, May 2018, pp. 121–29. PubMed, https://doi.org/10.1016/j.lfs.2018.03.056.
Ebbehøj, Niels E., et al. “Outbreak of Eczema and Rhinitis in a Group of Office Workers in Greenland.” International Journal of Circumpolar Health, vol. 74, 2015, p. 27919. PubMed, https://doi.org/10.3402/ijch.v74.27919.
Eslam, M., et al. “M039 RINGING SHOT: TINNITUS AS A POSSIBLE SIDE EFFECT TO THE NEW COVID19 MRNA VACCINE.” Annals of Allergy, Asthma & Immunology, vol. 127, no. 5, Nov. 2021, p. S69. www.annallergy.org, https://doi.org/10.1016/j.anai.2021.08.213.
Freedberg, Daniel E., et al. “Famotidine Use Is Associated With Improved Clinical Outcomes in Hospitalized COVID-19 Patients: A Propensity Score Matched Retrospective Cohort Study.” Gastroenterology, vol. 159, no. 3, Sept. 2020, pp. 1129-1131.e3. PubMed Central, https://doi.org/10.1053/j.gastro.2020.05.053.
Hagenow, Jens, and Holger Stark. “Histamine H₄ Receptor Antagonists: A New Approach for Tinnitus Treatment?” Recent Patents on CNS Drug Discovery, vol. 10, no. 1, 2015, pp. 6–9. PubMed, https://doi.org/10.2174/1574889810666150424161907.
Hovaguimian, Alexandra, and Christopher H. Gibbons. “Diagnosis and Treatment of Pain in Small Fiber Neuropathy.” Current Pain and Headache Reports, vol. 15, no. 3, June 2011, pp. 193–200. PubMed Central, https://doi.org/10.1007/s11916-011-0181-7.
Janowitz, Tobias, et al. “Famotidine Use and Quantitative Symptom Tracking for COVID-19 in Non-Hospitalised Patients: A Case Series.” Gut, vol. 69, no. 9, Sept. 2020, pp. 1592–97. PubMed Central, https://doi.org/10.1136/gutjnl-2020-321852.
Kazama, Itsuro. “Potential Prophylactic Efficacy of Mast Cell Stabilizers against COVID-19 Vaccine-Induced Anaphylaxis.” Clinical and Molecular Allergy, vol. 19, no. 1, Dec. 2021, p. 25. BioMed Central, https://doi.org/10.1186/s12948-021-00162-9.
Kempuraj, D., et al. “Luteolin Inhibits Myelin Basic Protein-Induced Human Mast Cell Activation and Mast Cell-Dependent Stimulation of Jurkat T Cells.” British Journal of Pharmacology, vol. 155, no. 7, Dec. 2008, pp. 1076–84. PubMed Central, https://doi.org/10.1038/bjp.2008.356.
Kohno, Ritsuko, et al. “Mast Cell Activation Disorder and Postural Orthostatic Tachycardia Syndrome: A Clinical Association.” Journal of the American Heart Association, vol. 10, no. 17, Sept. 2021, p. e021002. ahajournals.org (Atypon), https://doi.org/10.1161/JAHA.121.021002.
Lee, Jun-Kyoung, et al. “Association between Perfluorooctanoic Acid Exposure and Degranulation of Mast Cells in Allergic Inflammation.” Journal of Applied Toxicology: JAT, vol. 37, no. 5, May 2017, pp. 554–62. PubMed, https://doi.org/10.1002/jat.3389.
Liu, Z. Q., et al. “Vitamin D Contributes to Mast Cell Stabilization.” Allergy, vol. 72, no. 8, Aug. 2017, pp. 1184–92. PubMed, https://doi.org/10.1111/all.13110.
Novak, Peter, et al. “Mast Cell Disorders Are Associated with Decreased Cerebral Blood Flow and Small Fiber Neuropathy.” Annals of Allergy, Asthma & Immunology: Official Publication of the American College of Allergy, Asthma, & Immunology, vol. 128, no. 3, Mar. 2022, pp. 299-306.e1. PubMed, https://doi.org/10.1016/j.anai.2021.10.006.
Park, Hyo-Hyun, Soyoung Lee, Jae-Min Oh, et al. “Anti-Inflammatory Activity of Fisetin in Human Mast Cells (HMC-1).” Pharmacological Research, vol. 55, no. 1, Jan. 2007, pp. 31–37. PubMed, https://doi.org/10.1016/j.phrs.2006.10.002.
Park, Hyo-Hyun, Soyoung Lee, Hee-Young Son, et al. “Flavonoids Inhibit Histamine Release and Expression of Proinflammatory Cytokines in Mast Cells.” Archives of Pharmacal Research, vol. 31, no. 10, Oct. 2008, pp. 1303–11. PubMed, https://doi.org/10.1007/s12272-001-2110-5.
Parsons, I. T., et al. “Histamine, Mast Cell Tryptase and Post-Exercise Hypotension in Healthy and Collapsed Marathon Runners.” European Journal of Applied Physiology, vol. 121, no. 5, May 2021, pp. 1451–59. PubMed, https://doi.org/10.1007/s00421-021-04645-0.
Qiu, Shidong, et al. “Complement Activation Associated with Polysorbate 80 in Beagle Dogs.” International Immunopharmacology, vol. 15, no. 1, Jan. 2013, pp. 144–49. PubMed, https://doi.org/10.1016/j.intimp.2012.10.021.
Romero, Steven A., et al. “Mast Cell Degranulation and de Novo Histamine Formation Contribute to Sustained Postexercise Vasodilation in Humans.” Journal of Applied Physiology (Bethesda, Md.: 1985), vol. 122, no. 3, Mar. 2017, pp. 603–10. PubMed, https://doi.org/10.1152/japplphysiol.00633.2016.
Schaubschläger, W. W., et al. “Release of Mediators from Human Gastric Mucosa and Blood in Adverse Reactions to Benzoate.” International Archives of Allergy and Applied Immunology, vol. 96, no. 2, 1991, pp. 97–101. PubMed, https://doi.org/10.1159/000235478.
Sellaturay, Priya, et al. “Polyethylene Glycol (PEG) Is a Cause of Anaphylaxis to the Pfizer/BioNTech MRNA COVID‐19 Vaccine.” Clinical & Experimental Allergy, vol. 51, no. 6, June 2021, pp. 861–63. DOI.org (Crossref), https://doi.org/10.1111/cea.13874.
Small Fiber Neuropathy: MedlinePlus Genetics. https://medlineplus.gov/genetics/condition/small-fiber-neuropathy/. Accessed 3 May 2022.
Srivastav, Shival, et al. “Valsalva Maneuver.” StatPearls, StatPearls Publishing, 2022. PubMed, http://www.ncbi.nlm.nih.gov/books/NBK537248/.
Weng, Zuyi, et al. “Quercetin Is More Effective than Cromolyn in Blocking Human Mast Cell Cytokine Release and Inhibits Contact Dermatitis and Photosensitivity in Humans.” PloS One, vol. 7, no. 3, 2012, p. e33805. PubMed, https://doi.org/10.1371/journal.pone.0033805.
yang, huan, et al. “Famotidine Activates the Vagus Nerve Inflammatory Reflex to Attenuate Cytokine Storm.” Research Square, Apr. 2022, p. rs.3.rs-1493296. PubMed Central, https://doi.org/10.21203/rs.3.rs-1493296/v1.

