Key takeaways:
- Active T cells are an integral part of the body’s defense against pathogens and cancer.
- Chronic, persistent stimulation can cause T cells to go into a state of exhaustion, no longer functioning well enough to protect the body.
- T cell exhaustion can occur in cancer, immunotherapy, HIV, hepatitis C, and possibly long Covid/ME/CFS or vaccine injury.
- Genetic variants play a role in susceptibility to T cell exhaustion.
- In some cases, T cell exhaustion can be reversed by providing the T cells with the right nutrients and blocking the checkpoints.
What is T cell exhaustion, and why is it important?
T cells are a cornerstone of the immune system’s ability to respond to pathogens and cancer. They are a type of white blood cell (lymphocyte) that is part of the adaptive immune response.
T cell exhaustion is the state in which T cells lose their ability to function effectively. This can be caused by prolonged exposure to a persistent antigen, such as from a chronic infection, or due to fighting cancer.
Quick background on T cells:
T cells are a type of white blood cell, and there are multiple types of T cells:[ref]
- Cytotoxic T (CD8+) cells: These cells kill cells that are infected with bacteria or viruses. They also kill cancerous tumor cells. Cytotoxic T cells are also called CD8+ cells due to a CD8 receptor on their membranes.
- Helper T (CD4+) cells: Helper T cells don’t directly kill cells, but instead signal to cytotoxic T cells, B cells, and macrophages that a response is needed. They are also called CD4+ cells because they have a CD4 receptor.
- Regulatory T (T regs) cells: T regs help keep healthy cells from being attacked by the immune system and help to keep CD8+ T cells from being overactive.
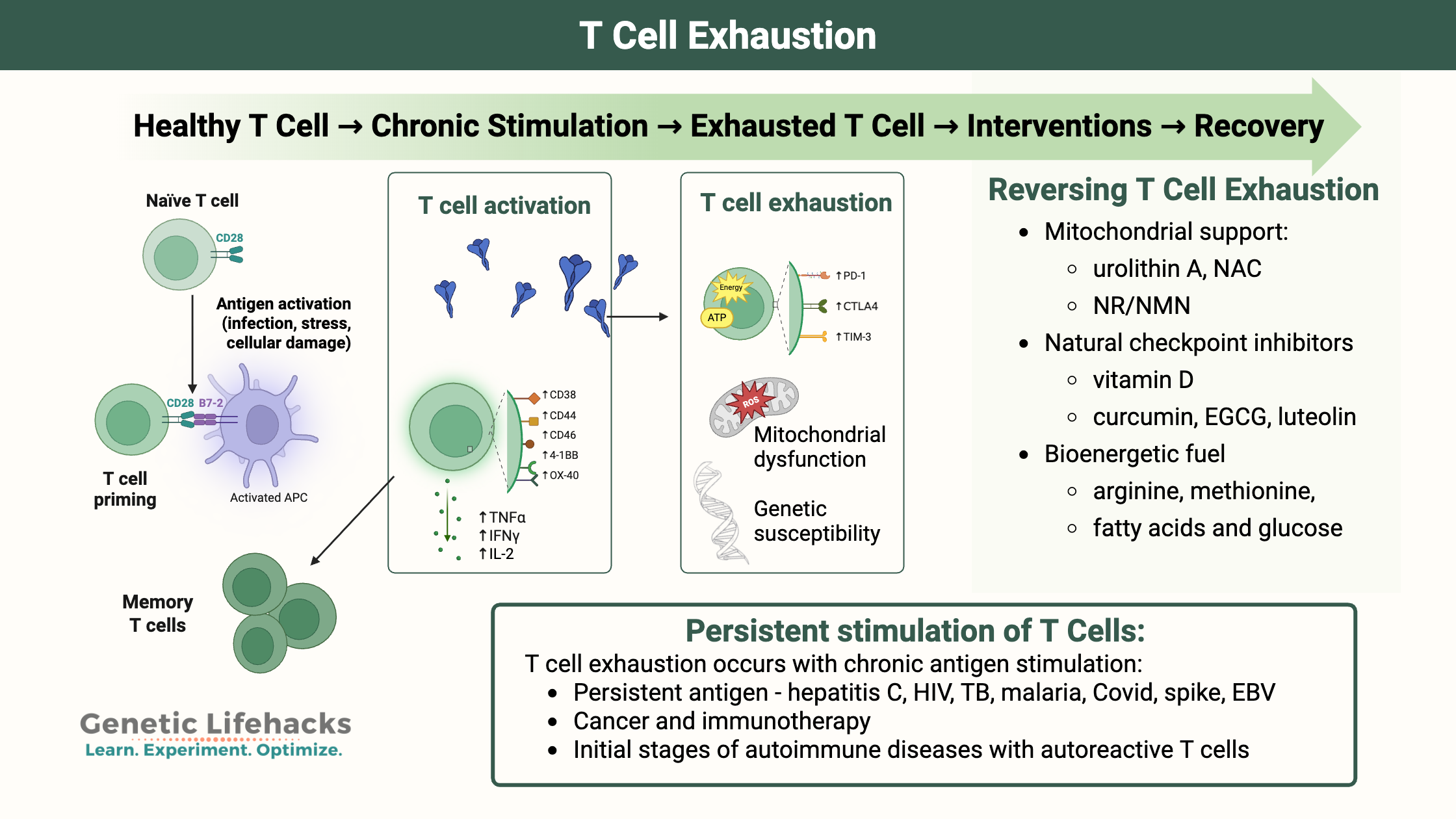
T cell activation:
Naive T cells are produced in the spleen and circulate until activated by antigens. Antigens are molecules from viruses, bacteria, allergens, or cancer cells, and they are presented on the surface of antigen-presenting cells.
Antigens activate naive T cells in a process requiring multiple signals to be present.
- Antigen presentation: Antigen-presenting cells are immune cells that process foreign substances (e.g. part of a virus, bacterium, or protein) and display the peptide on the cell surface, signaling danger to the rest of the immune system. Antigen-presenting cells include macrophages and B cells.[ref] CD4+ (helper) T cells recognize antigens presented by MHC class II molecules, which include HLA-DQ, HLA-DR, and HLA-DP. CD8+ (cytotoxic) T cells recognize antigens presented by MHC class I molecules.
- Costimulation: Along with the antigen, a costimulatory binding protein is also needed and usually found on the antigen-presenting cell. An interaction occurs between CD28 on the T cell and a ligand on the antigen-presenting cell (e.g. CD80, CD86).
- Inflammatory cytokines: Cytokine signaling is also involved. Interleukin 2 (IL-2) binds to the IL-2 receptor on the T cell.
- Glutamate receptors: A 2025 study adds in a little more detail on CD8+ T cell activation. Glutamate receptors (GluA3 and mGluR1) and glutamate transporters on T cells are involved in activation of the full CD8+ T cell response.[ref] (Glutamate receptor genes)
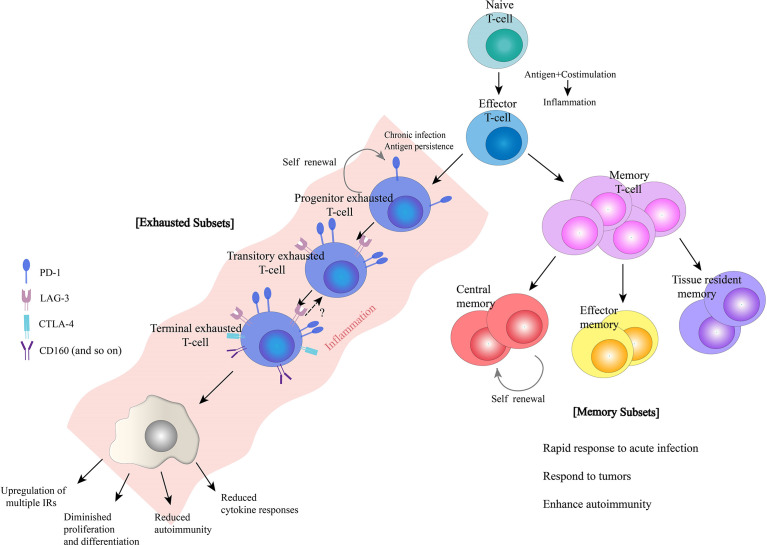
With all these signals, the naive T cell will undergo rapid cell division and differentiate into effector (active) T cells that fight off the pathogen.[ref] After the stimulus is gone, memory T cells develop and reside at low levels for life so that if the antigen is encountered again, a swift activation of the T cell immune response can happen.
T cell activation occurs over the course of 1-2 weeks when you have an acute infection or have had a vaccination.
T cell exhaustion: Causes and stages
T cell exhaustion is the term for the state in which T cells are no longer able to proliferate in response to antigen and costimulatory signals. Exhaustion is the result of prolonged antigenic stimulation along with inflammatory signals.
Much of the initial research about T cell exhaustion came out of HIV and hepatitis C research in the 90s. Persistent viral infections and prolonged antigen exposure eventually cause T cell exhaustion, leading to vulnerability to opportunistic pathogens, such as pneumonia or skin infections, in the case of HIV.[ref][ref]
Persistent stimulation:
T cell exhaustion occurs with chronic antigen stimulation, which can be caused by:
- Persistent, active infections, including hepatitis C, hepatitis B, HIV, tuberculosis, malaria, some long Covid[ref]
- Certain types of cancer or immunotherapy
- Repeated Covid mRNA vaccination[ref][ref][ref]
- Initial stages of some autoimmune diseases[ref]
Checkpoints upregulated to put the brakes on:
After chronic activation, the CD8+ T cells eventually move into an altered state, with a sustained upregulation of inhibitory checkpoint receptors, such as PD-1, TIM3, CTLA-4, and LAG-3, that put the brakes on the T cells’ activity (more details below). This state of T cell exhaustion then decreases your body’s ability to fight off cancer or infections. In cancer, T cell exhaustion can lead to immune escape, cancer progression, and metastasis.[ref][ref]
How long is ‘chronic’ when it comes to antigen exposure?
Studies in mice show that antigen exposure for 1-2 weeks allows activated T cells to turn into T memory cells, but antigen exposure for 2-4 weeks leads to T cell exhaustion.[ref]
While CD8+ T cell exhaustion is the primary problem and the focus of most studies, CD4+ T cells (T helper, T regs) can also suffer exhaustion and contribute to the problem in cancer.[ref]
Two hits: Persistent antigen plus hypoxia and mitochondrial dysfunction
A 2022 study showed that chronic stimulation alone wasn’t usually sufficient to cause T cell exhaustion in cancer. However, hypoxia, along with antigen stimulation, did cause T cell exhaustion, in conjunction with high levels of reactive oxygen species (ROS) from dysfunctional mitochondria. What’s interesting here is that the study showed that reducing hypoxia and reducing T cell ROS could reverse the exhaustion state.[ref]
In chronic infections, T cells lose mitochondrial energy function as a step towards the exhaustion state. High levels of ROS from mitochondrial dysfunction then cause cellular stress and damage to the T cells. Mitophagy is how damaged mitochondria are cleared out, and promoting mitophagy may help to rescue T cells before they become terminally exhausted.[ref]
Progressive state of T cell exhaustion:
T cell exhaustion seems like it is more like a dimmer switch than an automatic shutoff. There are levels of the state of exhaustion, and most of the time, there is still some residual activity and immune response from the T cells. They are just under-responsive.[ref]
Conditions with T cell exhaustion:
Cancer and cancer immunotherapy are the most common causes of T cell exhaustion. If you are fighting cancer, talk with your doctor about how to best support your immune system.
Persistent HIV or chronic hepatitis C viral infections may also cause T cell exhaustion – again, talk with your doctor for the best path forward here.
Beyond cancer and HIV, let’s take a look at other causes and conditions that involve CD8+ T cell exhaustion.
mRNA Vaccination and T cell exhaustion:
I mentioned above that long-term viral infections that cause persistent antigen production are a primary cause of T cell exhaustion. This raises the question: If mRNA vaccination causes persistent antigen production in some people, can it cause T cell exhaustion?
While initial estimates from the Infectious Disease Society of America estimated that the mRNA spike protein fragments would last for a few weeks, early clinical studies show that the spike protein circulates for a month to six months.[ref]
The Yale LISTEN study of patients with post-vaccination syndrome showed persistently elevated levels of antigen production for several years after vaccination. Participants with persistent antigen production had a significantly higher proportion of exhausted CD8+ T cells compared to controls. Post-vaccination syndrome in this study was characterized by “exercise intolerance, excessive fatigue, numbness, brain fog, neuropathy, insomnia, palpitations, myalgia, tinnitus or humming in ears, headache, burning sensations, and dizziness”. [ref – preprint]
A study of 16 adolescents and young adults with myocarditis after covid vaccination showed that they had “markedly elevated levels of full-length spike protein” that persisted unbound by antibodies.[ref]
To be clear: Most individuals who received Covid vaccinations will not have T cell exhaustion. There is heterogeneity in the amount of mRNA in the vaccine lots and in the immune response.
A Canadian study of 76 adults showed that the group as a whole did not have a statistically significant increase in T cell exhaustion after three or four covid shots. However, the elderly adults (mean age of 84) included in the study did show elevated T cell exhaustion markers following the third and fourth shots.[ref] Another study in cancer patients showed that some patients had a good response to the Covid booster, while approximately 30% of the cancer patients showed increased numbers of exhausted T cells after the third shot.[ref]
ME/CFS and T cell exhaustion:
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a debilitating disease that causes post-exertional malaise, cognitive changes, pain, flu-like symptoms, orthostatic intolerance, and fatigue. A 2024 study that analyzed T cell subsets found T cell exhaustion to be common in ME/CFS patients.[ref] Prior research had also shown that CD8+ T cells had metabolic reprogramming indicative of T cell exhaustion.[ref][ref][ref]
While it is easy to jump to the conclusion that T cell exhaustion could be the ultimate cause of ME/CFS, note that in addition to CD8+ T cell exhaustion, other changes in immune cells, including natural killer cells, have been found in ME/CFS. T cell exhaustion may be part of a broader picture or a consequence rather than a cause.
Long Covid and T cell exhaustion:
Long Covid is a heterogeneous condition, with some individuals reporting fatigue, post-exertional malaise, POTS, brain fog, and unrefreshing sleep, which are symptoms similar to ME/CFS.
Multiple studies have shown that a portion of long Covid patients have T cell changes that indicate T cell exhaustion. Moreover, one study showed that when treatment improved CD8+ T cell function, self-reported symptom severity decreased by over 50%. [ref][ref][ref]
Similar to ME/CFS, CD8+ T cell exhaustion may be just part of what is going on with the immune system changes in long Covid. The systemic elevation of inflammatory cytokines interacts with both T cell exhaustion and also mast cell activation, which then feeds back with continued release of inflammatory mediators from the activated mast cells. Mast cells are integral to T cell regulation.[ref][ref]
Notably, while CD8+ T cell exhaustion was seen in long Covid patients at 3 and 8 months post-infection, by 24 months, many of the long covid patients had T cells return to a more normal type and reported that their quality of life and health had improved.[ref]
COPD and lung disorders:
Studies show that individuals with COPD (chronic obstructive pulmonary disease) have altered levels of different types of T cells, and they are likely to have T cell exhaustion, T cell senescence, and reduced T cell levels. A study of lung tissue samples from COPD patients showed increased PD-1, indicating T cell exhaustion, compared to controls. A 2025 study showed that COPD lung tissue exhibited marked CD8+ T cell exhaustion markers. [ref][ref][ref]
When looking at how COPD develops, researchers found that there is a threshold reached with increasing T cell dysfunction prior to COPD being diagnosed. For many people, smoking is the triggering chronic stimulation event for COPD. Animal studies show that exposure to cigarette smoke significantly increases TIM-3, an inhibitor of active T cells, and thus promotes an exhausted T cell state along with immune dysfunction.[ref][ref]
Chronic allergic asthma, in animal studies, also causes T cell exhaustion.[ref]
Aging and T cell exhaustion:
With advanced age, immune function declines. Part of this decline involves a reduced T cell response due to cellular senescence in T cells, but this can also be accompanied by increased T cell exhaustion due to the combination of co-stimulatory molecules and mitochondrial dysfunction, causing increased ROS. [ref]
Hemochromatosis (iron overload):
Dysregulation of iron absorption can cause excess iron in the body, which is highly reactive. Excess iron gets stored in organs and tissues in hemochromatosis, which causes oxidative stress in the cells. A 2025 study shows that T cell exhaustion also occurs at a high rate in patients with hemochromatosis. While oxidative stress is likely an additive factor, the researchers also found that higher iron levels inhibited the enzymes that help turn off T cell activation.[ref]
Related article: Hemochromatosis and HFE mutations
Your genotype for rs1800562 is —. This means that your connected genetic data file shows a mutation related to hemochromatosis risk. Read the full article on iron and hemochromatosis here.
Consequences of T cell exhaustion:
T cell exhaustion poses problems for both immune function and overall health.
Drain on energy:
Constant T cell activation with antigens is energetically expensive for the body. The spleen is where T cells are activated, and the spleen is one of the most energy-demanding organs (higher even than the brain). Activated T cells require a lot of energy and use a large amount of glucose, amino acids, and fatty acids. (More on this in the Lifehacks section)[ref] [ref][ref]
Cancer survival:
In cancer immunotherapy, CD8+ T cell exhaustion leads to a poor response and reduced survival.
Viral reactivation:
In severe Covid patients with T cell exhaustion, reactivation of Epstein-Barr virus (EBV), cytomegalovirus, and herpes simplex virus has been noted.
Opportunistic infections:
Bacteria, fungi, and viruses are around and present in us all the time. Without active cytotoxic T cells, these pathogens can get out of control. For example, T cell exhaustion can lead to Candida overgrowth.[ref]
Autoimmune diseases:
T cell exhaustion is also found in some people with autoimmune diseases. Studies of patients who had long-term remission with lupus showed that T cell exhaustion may play a beneficial role in maintaining remission. Other studies also show better outcomes in autoimmunity with T cell exhaustion. In type 1 diabetes, CD8+ T cells can kill pancreatic islet β cells. T cell exhaustion in type 1 diabetes slows the rate of progression.[ref][ref]
However, there isn’t always a clear direction in T cell function. For example, in rheumatoid arthritis, reduced T cell function in synovial fluid suggests disease-specific differences or stage-dependent variations in T cell exhaustion. [ref]
Regulators of T cell response: Checkpoints prevent excess T cell activity
Active cytotoxic T cell response needs to be kept under control to prevent excess cell death or the targeting of healthy cells (e.g. prevent autoimmune diseases). It’s a balance between mounting a robust T cell response against cancer or viral infections while avoiding excessive or off-target responses.
Let’s take a look at the genes and receptors that regulate whether a CD8+ T cell is active or has moved into an exhausted state. This will give you the background to understand the genotype report section below.
PD-1 (Programmed cell death protein 1, PDCP1 gene):
PD-1 is a regulatory receptor on T cells that, when stimulated, sends an inhibitory signal to block T cell activation and reduce proliferation. Cancer cells can upregulate the ligand for PD-1, preventing T cell activation and allowing cancer cells to survive. In cancer treatment, PD-1 blockade can stimulate T cell responses to eliminate cancerous cells. Drugs that block the interaction between PD-1 and its ligands are a major class of cancer immunotherapy. However, PD-1 also plays an important role in keeping T cells under control in healthy organs. For example, in the pancreas, PD-1 prevents autoimmune attack on beta cells, thus preventing type 1 diabetes. [ref][ref]
TIM3 (T cell immunoglobulin and mucin domain-containing protein 3):
TIM3 is a receptor expressed on the surface of T cells and myeloid cells. It can act as an activating or inhibitory receptor, but in the context of activated T cells, TIM3 helps to inhibit an overactive inflammatory T cell response, particularly in autoimmune diseases. However, in chronic viral infection or cancer, TIM3 contributes to T cell exhaustion.[ref]

CTLA4 (Cytotoxic T-lymphocyte-associated protein 4):
CTLA4 is another negative regulator of the immune system. Again, it is like a brake on the immune response when it is expressed on activated T cells. Higher levels of CTLA4 are associated with an increased risk of T cell exhaustion and worse cancer prognosis.[ref] Lower levels of CTLA4 are associated with an increased risk of some autoimmune diseases, although it isn’t always straightforward.[ref][ref]
LAG-3 (Lymphocyte-activation gene 3):
LAG-3 is a cell surface receptor on T cells that regulates T cell response, acting as a brake or checkpoint. This receptor is expressed on both CD4+ and CD8+ T cells. However, excessive LAG-3 activation can cause T cell exhaustion. LAG-3 inhibitors are an active area of research for cancer immunotherapy, and one LAG-3 inhibitor has been approved for melanoma in combination with other immunotherapies.[ref][ref]
TOX:
TOX is a transcription factor that turns on and off genes in mature CD8+ T cells. Researchers in 2019 discovered that TOX is a critical part of the epigenetic changes that occur in exhausted T cells. Essentially, the TOX transcription factor turns on the inhibitors, LAG-3 and PD-1. TOX also increases IL-10.[ref][ref]
IL-10 (interleukin 10):
IL-10 is a regulatory cytokine that puts the brakes on the immune response, preventing excess tissue damage during inflammation. Elevated IL-10 levels are associated with T cell exhaustion, and blockade of the IL-10 receptor can restore T cell function in a viral infection. However, IL-10’s role in cancer-related T cell exhaustion appears to differ, and reduced IL-10 levels may impair cancer immunotherapy outcomes.[ref][ref]
ADRB1 (β1-adrenergic receptor):
β1-adrenergic signaling through ADRB1 has recently been identified as having a role in T cell exhaustion. The ADRB1 receptor is activated by stress hormones, such as norepinephrine. Chronic ADRB1 stimulation in T cells can promote exhaustion by increasing expression of inhibitory receptors. Research shows that beta blockers, which are medications that block the ADRB1 receptor, may have potential in T cell exhaustion in cancer immunotherapy.[ref]
Epigenetic changes:
In addition to the checkpoint changes that drive T cell exhaustion, epigenetic changes occur that can eventually prevent T cells from being reinvigorated by checkpoint inhibitors.[ref] While epigenetic changes are not covered in depth here, they represent an important additional dimension of T cell exhaustion.
Let’s shift gears and look at the genetic variants that can increase the odds of T cell exhaustion at a lower threshold.
Genotype Report: T cell Exhaustion
Access this content:
An active subscription is required to access this content.
Lifehacks: Solutions for T cell Exhaustion
This section covers some of the natural solutions that may help with T cell exhaustion.
Reversing T cell exhaustion is accomplished by blocking the checkpoints, such as TIM3, PD-1, and CTLA4, while also providing the metabolism-related compounds needed for the T cells to thrive energetically and have healthy mitochondrial function.[ref]
A three-pronged approach to addressing T cell exhaustion with natural supplements includes:[ref]
- Promote mitochondrial function and reduce ROS in T cells
- Natural checkpoint inhibitors to prevent exhaustion state
- Meet the bioenergetic needs of T cells
1) Promoting mitochondrial function:
To reiterate – T cell exhaustion involves continued antigen exposure along with excessive ROS from mitochondrial dysfunction. Mitochondrial support may help reverse early-stage T cell exhaustion.[ref][ref]
Urolithin A:
A 2025 study shows that supplemental urolithin A in the form of MitoTempo increased mitochondrial health and specifically moved T cells to a less exhausted state in older adults. This study builds on previous research demonstrating improved mitochondrial function with urolithin A supplementation.[ref]
Related article: Urolithin A Supplementation and Diet
Access this content:
An active subscription is required to access this content.
Related articles and topics:
ME/CFS: Myalgic Encephalomyelitis / Chronic Fatigue Syndrome (and Long Covid?) Genes
References:
Ahn, So Yeon, et al. “Chronic Allergic Asthma Induces T-Cell Exhaustion and Impairs Virus Clearance in Mice.” Respiratory Research, vol. 24, 2023, p. 160. PubMed Central, https://doi.org/10.1186/s12931-023-02448-9.
Alavi, Sara, et al. “Nicotinamide Inhibits T Cell Exhaustion and Increases Differentiation of CD8 Effector T Cells.” Cancers, vol. 14, no. 2, Jan. 2022, p. 323. PubMed Central, https://doi.org/10.3390/cancers14020323.
Alberts, Bruce, et al. “Helper T Cells and Lymphocyte Activation.” Molecular Biology of the Cell. 4th Edition, Garland Science, 2002. www.ncbi.nlm.nih.gov, https://www.ncbi.nlm.nih.gov/books/NBK26827/.
Bandiwadekar, Chaitra, et al. “Attenuation of Malignant Phenotype of Glioblastoma Following a Short Course of the Pro-Oxidant Combination of Resveratrol and Copper.” BJC Reports, vol. 3, no. 1, Sept. 2025, p. 68. www.nature.com, https://doi.org/10.1038/s44276-025-00177-8.
Belk, Julia A., et al. “Epigenetic Regulation of T Cell Exhaustion.” Nature Immunology, vol. 23, no. 6, June 2022, pp. 848–60. www.nature.com, https://doi.org/10.1038/s41590-022-01224-z.
Benitez Fuentes, Javier David, et al. “Evidence of Exhausted Lymphocytes after the Third Anti-SARS-CoV-2 Vaccine Dose in Cancer Patients.” Frontiers in Oncology, vol. 12, Dec. 2022, p. 975980. PubMed Central, https://doi.org/10.3389/fonc.2022.975980.
Benoit, Jenna M., et al. “No Evidence of Immune Exhaustion after Repeated SARS-CoV-2 Vaccination in Vulnerable and Healthy Populations.” Nature Communications, vol. 16, no. 1, June 2025, p. 5219. www.nature.com, https://doi.org/10.1038/s41467-025-60216-3.
Bhattacharjee, Bornali, et al. “Immunological and Antigenic Signatures Associated with Chronic Illnesses after COVID-19 Vaccination.” 18 Feb. 2025. Allergy and Immunology, https://doi.org/10.1101/2025.02.18.25322379.
Blank, Christian U., et al. “Defining ‘T Cell Exhaustion.’” Nature Reviews Immunology, vol. 19, no. 11, Nov. 2019, pp. 665–74. www.nature.com, https://doi.org/10.1038/s41577-019-0221-9.
Boros, László G., et al. “Long‐lasting, Biochemically Modified mRNA, and Its Frameshifted Recombinant Spike Proteins in Human Tissues and Circulation after COVID ‐19 Vaccination.” Pharmacology Research & Perspectives, vol. 12, no. 3, June 2024, p. e1218. DOI.org (Crossref), https://doi.org/10.1002/prp2.1218.
Canaria, D. Alejandro, et al. “Tox Induces T Cell IL-10 Production in a BATF-Dependent Manner.” Frontiers in Immunology, vol. 14, Nov. 2023. Frontiers, https://doi.org/10.3389/fimmu.2023.1275423.
Chen, Mi, et al. “Targeting T-Cell Aging to Remodel the Aging Immune System and Revitalize Geriatric Immunotherapy.” Aging and Disease, Mar. 2025, p. 0. www.aginganddisease.org, https://doi.org/10.14336/AD.2025.0061.
Chen-Camaño, Roderick, et al. “T-Cell Exhaustion in COVID-19: What Do We Know?” Frontiers in Immunology, vol. 16, Oct. 2025, p. 1678149. PubMed Central, https://doi.org/10.3389/fimmu.2025.1678149.
Davis, Lawrence. Efficiency of the Human Body. openoregon.pressbooks.pub, https://openoregon.pressbooks.pub/bodyphysics/chapter/human-metabolism/. Accessed 19 Nov. 2025.
Delmas, Dominique, et al. “PD-1/PD-L1 Checkpoints and Resveratrol: A Controversial New Way for a Therapeutic Strategy.” Cancers, vol. 13, no. 18, Sept. 2021, p. 4509. PubMed Central, https://doi.org/10.3390/cancers13184509.
Denk, Dominic, et al. “Effect of the Mitophagy Inducer Urolithin A on Age-Related Immune Decline: A Randomized, Placebo-Controlled Trial.” Nature Aging, vol. 5, no. 11, Nov. 2025, pp. 2309–22. www.nature.com, https://doi.org/10.1038/s43587-025-00996-x.
Falcone, Marika, and Georgia Fousteri. “Role of the PD-1/PD-L1 Dyad in the Maintenance of Pancreatic Immune Tolerance for Prevention of Type 1 Diabetes.” Frontiers in Endocrinology, vol. 11, Aug. 2020, p. 569. PubMed Central, https://doi.org/10.3389/fendo.2020.00569.
Gao, Zhanyan, et al. “T-Cell Exhaustion in Immune-Mediated Inflammatory Diseases: New Implications for Immunotherapy.” Frontiers in Immunology, vol. 13, Sept. 2022. Frontiers, https://doi.org/10.3389/fimmu.2022.977394.
———. “T-Cell Exhaustion in Immune-Mediated Inflammatory Diseases: New Implications for Immunotherapy.” Frontiers in Immunology, vol. 13, Sept. 2022, p. 977394. PubMed Central, https://doi.org/10.3389/fimmu.2022.977394.
Geiger, Roger, et al. “L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-Tumor Activity.” Cell, vol. 167, no. 3, Oct. 2016, pp. 829-842.e13. PubMed Central, https://doi.org/10.1016/j.cell.2016.09.031.
Gil, Anna, et al. “Identification of CD8 T-Cell Dysfunction Associated with Symptoms in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) and Long COVID and Treatment with a Nebulized Antioxidant/Anti-Pathogen Agent in a Retrospective Case Series.” Brain, Behavior, & Immunity – Health, vol. 36, Dec. 2023, p. 100720. PubMed Central, https://doi.org/10.1016/j.bbih.2023.100720.
Graydon, Colin G., et al. “LAG3’s Enigmatic Mechanism of Action.” Frontiers in Immunology, vol. 11, Jan. 2021, p. 615317. PubMed Central, https://doi.org/10.3389/fimmu.2020.615317.
Han, Chenfeng, et al. “Cystine Deprivation Triggers CD36-Mediated Ferroptosis and Dysfunction of Tumor Infiltrating CD8+ T Cells.” Cell Death & Disease, vol. 15, no. 2, Feb. 2024, p. 145. PubMed, https://doi.org/10.1038/s41419-024-06503-1.
Hashemi, Mohammad, et al. “Association between PD-1 and PD-L1 Polymorphisms and the Risk of Cancer: A Meta-Analysis of Case-Control Studies.” Cancers, vol. 11, no. 8, Aug. 2019, p. 1150. PubMed Central, https://doi.org/10.3390/cancers11081150.
Hirata, So-ichiro, et al. “Vitamin B1 Supports the Differentiation of T Cells through TGF-β Superfamily Production in Thymic Stromal Cells.” iScience, vol. 23, no. 9, Sept. 2020, p. 101426. ScienceDirect, https://doi.org/10.1016/j.isci.2020.101426.
Hosseini, Arezoo, et al. “CTLA-4: From Mechanism to Autoimmune Therapy.” International Immunopharmacology, vol. 80, Mar. 2020, p. 106221. ScienceDirect, https://doi.org/10.1016/j.intimp.2020.106221.
Hu, Yang, et al. “Reversal of T-Cell Exhaustion: Mechanisms and Synergistic Approaches.” International Immunopharmacology, vol. 138, Sept. 2024, p. 112571. ScienceDirect, https://doi.org/10.1016/j.intimp.2024.112571.
Iu, David S., et al. “Transcriptional Reprogramming Primes CD8+ T Cells toward Exhaustion in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome.” Proceedings of the National Academy of Sciences, vol. 121, no. 50, Dec. 2024, p. e2415119121. DOI.org (Crossref), https://doi.org/10.1073/pnas.2415119121.
Jiang, Ze-Bo, et al. “Luteolin and Its Derivative Apigenin Suppress the Inducible PD-L1 Expression to Improve Anti-Tumor Immunity in KRAS-Mutant Lung Cancer.” Cancer Letters, vol. 515, Sept. 2021, pp. 36–48. PubMed, https://doi.org/10.1016/j.canlet.2021.05.019.
Kalathil, Suresh Gopi, et al. “T-Regulatory Cells and Programmed Death 1+ T Cells Contribute to Effector T-Cell Dysfunction in Patients with Chronic Obstructive Pulmonary Disease.” American Journal of Respiratory and Critical Care Medicine, vol. 190, no. 1, July 2014, pp. 40–50. DOI.org (Crossref), https://doi.org/10.1164/rccm.201312-2293OC.
Kang, Kuan, et al. “T Cell Exhaustion in Human Cancers.” Biochimica et Biophysica Acta (BBA) – Reviews on Cancer, vol. 1879, no. 5, Sept. 2024, p. 189162. ScienceDirect, https://doi.org/10.1016/j.bbcan.2024.189162.
Ke, Junyi, et al. “Integrated Bioinformatic Analysis and Experimental Validation for Exploring the Key Immune Checkpoint of COPD.” Gene, vol. 927, Nov. 2024, p. 148711. ScienceDirect, https://doi.org/10.1016/j.gene.2024.148711.
Khan, Omar, et al. “TOX Transcriptionally and Epigenetically Programs CD8+ T Cell Exhaustion.” Nature, vol. 571, no. 7764, July 2019, pp. 211–18. www.nature.com, https://doi.org/10.1038/s41586-019-1325-x.
Klocke, Katrin, et al. “Induction of Autoimmune Disease by Deletion of CTLA-4 in Mice in Adulthood.” Proceedings of the National Academy of Sciences, vol. 113, no. 17, Apr. 2016. DOI.org (Crossref), https://doi.org/10.1073/pnas.1603892113.
Lee, Juwon, et al. “Phytochemicals in Cancer Immune Checkpoint Inhibitor Therapy.” Biomolecules, vol. 11, no. 8, July 2021, p. 1107. PubMed Central, https://doi.org/10.3390/biom11081107.
Li, Fei, et al. “Mitochondrial Metabolism in T-Cell Exhaustion.” International Journal of Molecular Sciences, vol. 26, no. 15, July 2025, p. 7400. PubMed Central, https://doi.org/10.3390/ijms26157400.
Li, Peng, et al. “1α,25(OH)2D3 Reverses Exhaustion and Enhances Antitumor Immunity of Human Cytotoxic T Cells.” Journal for Immunotherapy of Cancer, vol. 10, no. 3, Mar. 2022, p. e003477. PubMed, https://doi.org/10.1136/jitc-2021-003477.
Mandarano, Alexandra H., et al. “Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients Exhibit Altered T Cell Metabolism and Cytokine Associations.” The Journal of Clinical Investigation, vol. 130, no. 3, Mar. 2020, pp. 1491–505. PubMed, https://doi.org/10.1172/JCI132185.
Maya, Jessica, et al. “Altered Fatty Acid Oxidation in Lymphocyte Populations of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome.” International Journal of Molecular Sciences, vol. 24, no. 3, Jan. 2023, p. 2010. PubMed, https://doi.org/10.3390/ijms24032010.
McKendry, Richard T., et al. “Dysregulation of Antiviral Function of CD8+ T Cells in the Chronic Obstructive Pulmonary Disease Lung. Role of the PD-1–PD-L1 Axis.” American Journal of Respiratory and Critical Care Medicine, vol. 193, no. 6, Mar. 2016, pp. 642–51. DOI.org (Crossref), https://doi.org/10.1164/rccm.201504-0782OC.
Mellinghoff, Sibylle C., et al. “T-Cells of Invasive Candidiasis Patients Show Patterns of T-Cell-Exhaustion Suggesting Checkpoint Blockade as Treatment Option.” Journal of Infection, vol. 84, no. 2, Feb. 2022, pp. 237–47. ScienceDirect, https://doi.org/10.1016/j.jinf.2021.12.009.
Miggelbrink, Alexandra M., et al. “CD4 T-Cell Exhaustion: Does It Exist and What Are Its Roles in Cancer?” Clinical Cancer Research, vol. 27, no. 21, Nov. 2021, pp. 5742–52. PubMed Central, https://doi.org/10.1158/1078-0432.CCR-21-0206.
Phetsouphanh, Chansavath, et al. “Improvement of Immune Dysregulation in Individuals with Long COVID at 24-Months Following SARS-CoV-2 Infection.” Nature Communications, vol. 15, no. 1, Apr. 2024, p. 3315. PubMed, https://doi.org/10.1038/s41467-024-47720-8.
Richter, Felix Clemens, et al. “Take My Breath Away—Mitochondrial Dysfunction Drives CD8+ T Cell Exhaustion.” Genes & Immunity, vol. 25, no. 1, Feb. 2024, pp. 4–6. www.nature.com, https://doi.org/10.1038/s41435-023-00233-8.
Scharping, Nicole E., et al. “Mitochondrial Stress Induced by Continuous Stimulation under Hypoxia Rapidly Drives T Cell Exhaustion.” Nature Immunology, vol. 22, no. 2, Feb. 2021, pp. 205–15. www.nature.com, https://doi.org/10.1038/s41590-020-00834-9.
Sharma, Piyush, et al. “Early Methionine Availability Attenuates T Cell Exhaustion.” Nature Immunology, vol. 26, no. 8, Aug. 2025, pp. 1384–96. www.nature.com, https://doi.org/10.1038/s41590-025-02223-6.
Soudeyns, H., et al. “Initiation of Antiretroviral Therapy during Primary HIV-1 Infection Induces Rapid Stabilization of the T-Cell Receptor Beta Chain Repertoire and Reduces the Level of T-Cell Oligoclonality.” Blood, vol. 95, no. 5, Mar. 2000, pp. 1743–51. PubMed.
Sun, Yujing, et al. “Immunometabolic Changes and Potential Biomarkers in CFS Peripheral Immune Cells Revealed by Single-Cell RNA Sequencing.” Journal of Translational Medicine, vol. 22, Oct. 2024, p. 925. PubMed Central, https://doi.org/10.1186/s12967-024-05710-w.
Wang, Changli, et al. “The Role of TIM-3 in Sepsis: A Promising Target for Immunotherapy?” Frontiers in Immunology, vol. 15, Mar. 2024, p. 1328667. PubMed Central, https://doi.org/10.3389/fimmu.2024.1328667.
Wherry, E. John, and Makoto Kurachi. “Molecular and Cellular Insights into T Cell Exhaustion.” Nature Reviews. Immunology, vol. 15, no. 8, Aug. 2015, pp. 486–99. PubMed Central, https://doi.org/10.1038/nri3862.
Wiedeman, Alice E., et al. “Autoreactive CD8+ T Cell Exhaustion Distinguishes Subjects with Slow Type 1 Diabetes Progression.” The Journal of Clinical Investigation, vol. 130, no. 1, pp. 480–90. PubMed Central, https://doi.org/10.1172/JCI126595. Accessed 19 Nov. 2025.
Wu, Yahua, et al. “The Identification of Genes Associated T-Cell Exhaustion and Construction of Prognostic Signature to Predict Immunotherapy Response in Lung Adenocarcinoma.” Scientific Reports, vol. 13, no. 1, Aug. 2023, p. 13415. www.nature.com, https://doi.org/10.1038/s41598-023-40662-z.
Yin, Kailin, et al. “Long COVID Manifests with T Cell Dysregulation, Inflammation and an Uncoordinated Adaptive Immune Response to SARS-CoV-2.” Nature Immunology, vol. 25, no. 2, Feb. 2024, pp. 218–25. PubMed, https://doi.org/10.1038/s41590-023-01724-6.
Yonker, Lael M., et al. “Circulating Spike Protein Detected in Post–COVID-19 mRNA Vaccine Myocarditis.” Circulation, vol. 147, no. 11, Mar. 2023, pp. 867–76. PubMed Central, https://doi.org/10.1161/CIRCULATIONAHA.122.061025.