Key takeaways:
~ The coagulation cascade is a complex series of steps that occurs to form a blood clot.
~ Certain genetic variants (SNPs) can increase the clotting cascade and put you at risk of deep vein thrombosis or heart attacks.
~ Blood clots can also be caused by infections since inflammatory cytokines released during the immune response can trigger clotting.
~ Understanding your genetic propensity towards clotting can help you know what symptoms to look for and when to seek help.
Overview of how blood clots form:
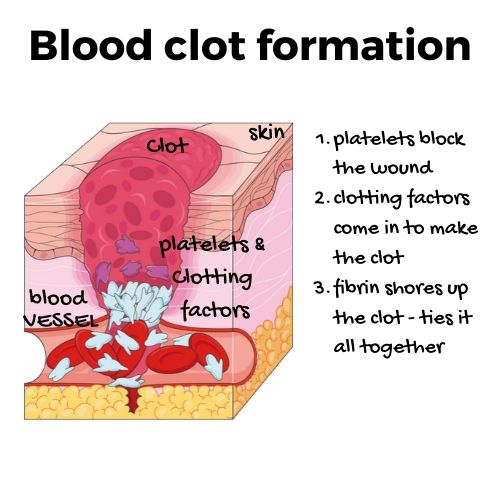
When you get a cut or tear in a blood vessel, the first action is that the platelets flowing through the bloodstream jump into the gap. They seal up the broken blood vessels preventing too much blood loss. The platelets must link up and connect with the edges of the cut and then with each other.
Next up is the coagulation cascade. This action is where various coagulation factors become activated. The coagulation factors are proteins that are also called clotting factors. They are inactive when flowing through the bloodstream (so they don’t clog everything up). When the coagulation factors activate, they can quickly form a blood clot.
The final step in clotting is fibrin’s ability to shore up the clot. Next, the body starts breaking down the clot through a process called fibrinolysis. It becomes a balance of adding fibrin and breaking it down.
Going a little deeper: More about platelets
Platelets form from megakaryocyte cells in the bone marrow and lungs. Tiny in size, platelets only survive for 7 to 10 days. These little clot formers have no nucleus (thus, no nuclear DNA). They contain mitochondria, along with granules containing different chemical signals and factors needed for coagulation.
When platelets are zipping around throughout the body, they are not ‘sticky’ nor clumping together. Instead, they are in an inactivated form that allows them to flow freely through your veins.
On the cell membrane of platelets are receptors that link to collagen. When a blood vessel tears, platelets bind to the collagen exposed at the rupture site. This binding causes the platelets to change shape and secrete chemical signals, initiating the coagulation cascade.[ref]
In addition to platelets binding to exposed collagen, another molecule called the von Willebrand factor (VWF) can also bind to both collagen and the platelet. In a sense, the von Willebrand factor forms a bridge between platelets and the surface of the blood vessel, and your circulating level of von Willebrand factor is important in how long it takes you to clot.[ref]
When platelets bind to either collagen or the von Willebrand factor, the activation of a signaling cascade occurs, releasing granules from the platelets. The release includes fibrinogen, ADP, serotonin, as well as other molecules, and the activation of COX-1 (which is inhibited by aspirin).[ref]
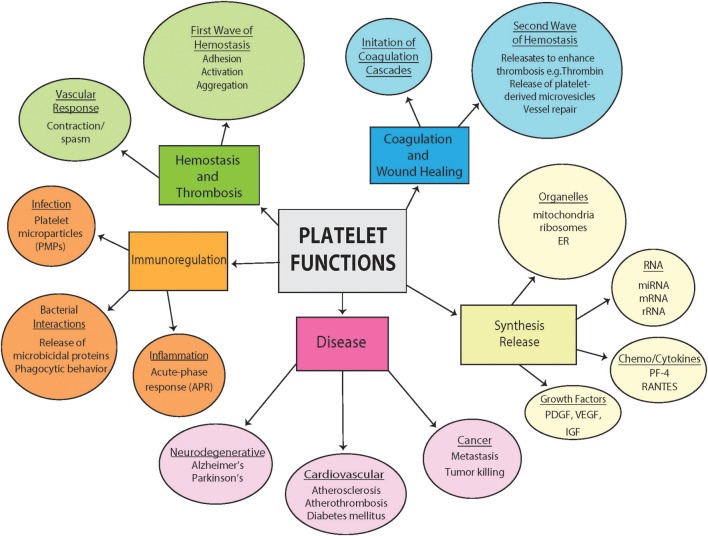
The figure below depicts all platelet functions – from coagulation and wound healing to immune functions. CC Image[ref]
So how many of these little guys are zipping around the body? A healthy adult has between 150,000 and 450,000 platelets per microliter of blood.
- A low platelet count (less than 150,000/ul) is known as thrombocytopenia
- A high platelet count (greater than 450,000/ul) is called thrombocytosis
Coagulation Cascade:
Let’s dig a little deeper into the coagulation cascade so that the genetic variants listed below in the genotype report make a little more sense. Basically – this is a whole cascade of events that happens quickly and results in the formation of cross-linked fibrin, which forms a mesh to keep the clot together.
The coagulation factors are numbered and referred to with Roman numerals -for example, factor five is written as factor V. Coagulation factors are found both in the plasma (e.g., factor IV) and the endothelial cells that form blood vessels (factors III and VIII).
The coagulation cascade can be divided into two pathways: the extrinsic and intrinsic pathways[ref]
- In the extrinsic pathway, tissue factor activates factor VII. The activated factor VII then activates factor X and factor IX. Factor X binds to activated factor V (along with a calcium molecule), and this causes the activation of prothrombin into thrombin. Thrombin converts fibrinogen into fibrin.
- The intrinsic pathway is also called the contact pathway. After thrombin formation, factor XI activates, causing further activation of factor X and factor IX. Factor X then binds with activated factor V, creating more thrombin. (This is a minor player in the clotting game.)
Finally, all of this results in a fibrin polymer mesh created through the involvement of activated factor XIII. This mesh then holds the clot together, binding the platelets with the other factors.
After the clot forms, it balances out with the activation of plasminogen, an enzyme that breaks down some of the fibrin in the clot. Basically, there is a balancing act of forming the clot and breaking down the clot while the wound heals.
D-dimer releases as clots break down and are often measured in the blood to determine if someone has a blood clot.
Thrombosis: blood clots forming inside a blood vessel
While it is easy to visualize the blood clotting process when you think of a paper cut on your finger, this same process can take place in blood vessels deep inside you. Instead of a cut, there are a variety of different ‘insults’ to the blood vessel that can cause a clot to form.
So what is the big deal about ‘thrombosis’? Why do we care about blood clotting in a blood vessel?
The majority of heart attacks and about 80% of strokes are caused by blood clots.
Blood clots forming deep in the leg are called deep vein thrombosis (DVT). The clot can block blood from flowing past it, or it could break free and travel through the veins, lodging in the lungs, and causing a pulmonary embolism. Anticoagulant medications treat blood clots but come with risks of excess bleeding. In all, the case-fatality rates for DVT and pulmonary embolisms are between 5 and 10% over the course of 30 days.[ref]
Thrombosis due to viral or bacterial infections:
Blood clots can also form due to an infection caused by bacteria or viruses.
Currently, this is in the news with reports of people getting blood clots due to SARS-CoV2 infections.[ref][ref][ref]
Blood clots, though, aren’t unique to COVID-19. Instead, they are a risk factor in many severe infectious diseases, including influenza and staph infections. In fact, people with severe complications from the H1N1 strain of the flu were at an 18 to 23-fold greater risk for blood clots.[ref][ref]
When the body actively fights off an infection, a lot of proinflammatory cytokines (IL-6, TNF-alpha, IL-1, IFNγ) are released. These cytokines cause leukocytes (white blood cells) and the surrounding epithelial cells to release procoagulants, such as tissue factors. These procoagulants can tip the body towards too much coagulation, with platelets clumping together. It can cause small clots to form, plugging up small blood vessels. The kidneys, liver, lungs, and brain are particularly vulnerable.[ref][ref]
Infection by bacteria or viruses increases the risk of thrombosis by 2 to 20-fold. The risk is greatest when the infection is active – and in the first few weeks after the infection. Complications from clots, such as strokes, are common in the first three days after respiratory infections and urinary tract infections.[ref]
ARDS (acute respiratory distress syndrome) is a severe complication of pneumonia due to viral or bacterial infections. Research shows that bacterial pneumonia causes “increased platelet aggregation, pulmonary microvascular thrombosis, endothelial damage, and hyper-inflammatory cytokine responses.”[ref]
DIC – disseminated intravascular coagulation:
In severe infections, such as sepsis, a condition called disseminated intravascular coagulation (DIC) can occur. DIC causes increased coagulation in the small blood vessels and micro-clots to form in various organs.[ref]
The increased number of clots then causes a depletion of platelets and clotting factors. So, on the one hand, you have a lot of little blood clots, but on the other hand, you eventually increase bleeding risk due to the decreased platelets and decreased fibrinogen. These clots also lead to elevated levels of D-dimer, which is released when the fibrin clots break down.
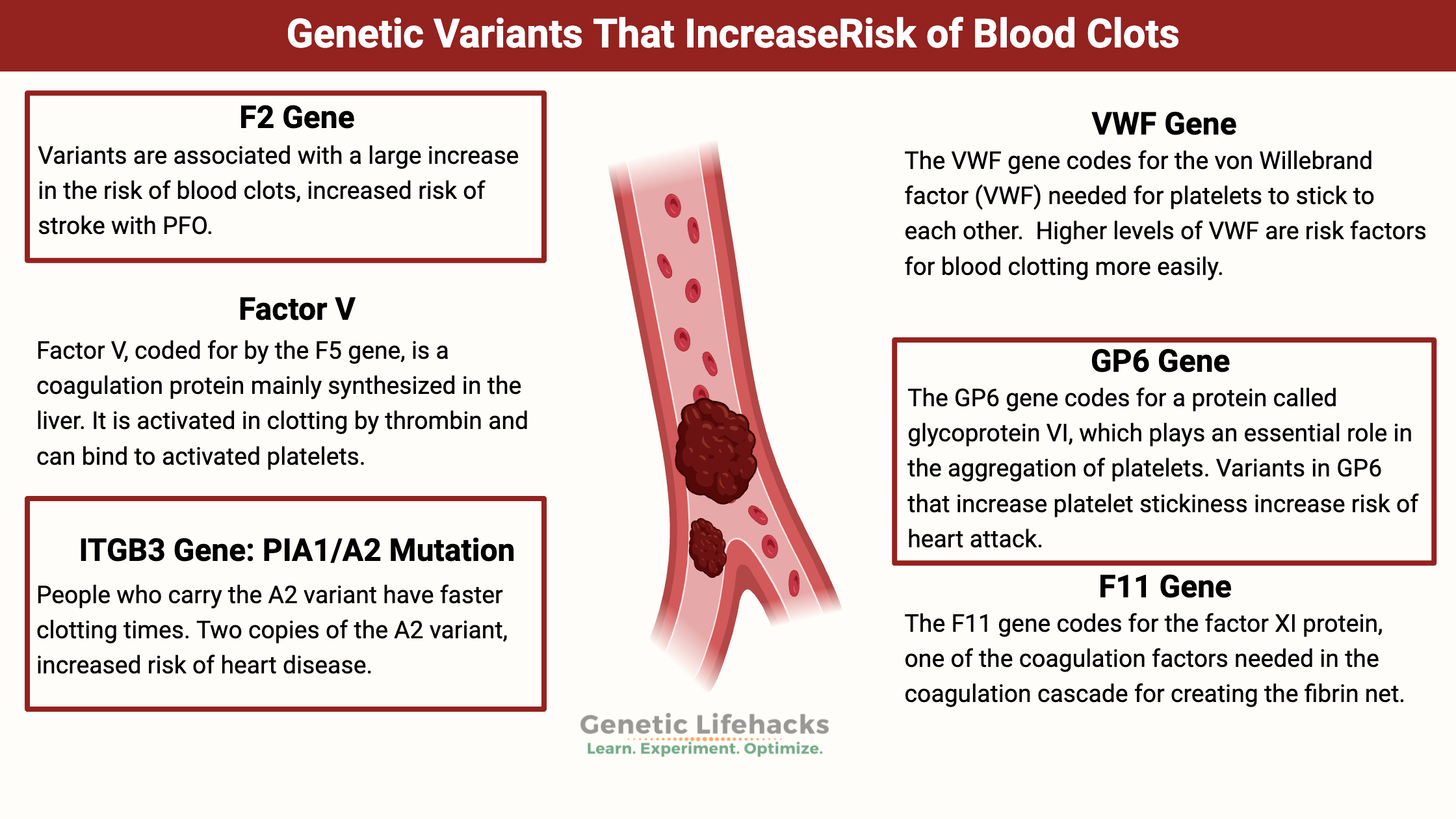
Blood Clots Genotype Report:
Lifehacks:
Take the information about your blood clot genetic risk factors as a ‘heads up’.
- Don’t ignore the signs of a blood clot.
- Symptoms of a blood clot in your arm or leg can include swelling, pain, redness, and warmth.
- If you suspect a clot, head to the doctor for an assessment.
- While the risk of blood clots increases with age, people genetically prone to clots can get one at any age.
Sugar alcohols:
Several studies have recently shown that sugar alcohols, such as erythritol, increase platelet stickiness and clot formation. People in the top quartile (top 25% ) of erythritol consumers are at an increased risk of heart attacks. Interestingly, a study showed that consuming the equivalent of an erythritol-sweetened beverage increased platelet reactivity for more than two days.[ref] A 2024 study showed the mechanism of action for increased platelet reactivity, clotting, and erythritol.[ref] While some question the link between sugar alcohols and platelet reactivity, it is something to consider if you have a genetically increased risk of clots.[ref]
Natural supplements that act as mild blood thinners:
The rest of this article is for Genetic Lifehacks members only. Consider joining today to see the rest of this article.
Related Articles and Topics:
References:
Arsov, Todor, et al. “Factor V Leiden Is Associated with Higher Risk of Deep Venous Thrombosis of Large Blood Vessels.” Croatian Medical Journal, vol. 47, no. 3, June 2006, pp. 433–39. PubMed Central, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2080416/.
Campello, Elena, et al. “Diagnosis and Management of Factor V Leiden.” Expert Review of Hematology, vol. 9, no. 12, Dec. 2016, pp. 1139–49. PubMed, https://doi.org/10.1080/17474086.2016.1249364.
El-Galaly, Tarec C., et al. “Single Nucleotide Polymorphisms and the Risk of Venous Thrombosis: Results from a Danish Case-Cohort Study.” British Journal of Haematology, vol. 160, no. 6, Mar. 2013, pp. 838–41. PubMed, https://doi.org/10.1111/bjh.12132.
Floyd, Christopher N., et al. “The PlA1/A2 Polymorphism of Glycoprotein IIIa as a Risk Factor for Myocardial Infarction: A Meta-Analysis.” PLOS ONE, vol. 9, no. 7, July 2014, p. e101518. PLoS Journals, https://doi.org/10.1371/journal.pone.0101518.
Fountain, John H., and Sarah L. Lappin. “Physiology, Platelet.” StatPearls, StatPearls Publishing, 2022. PubMed, http://www.ncbi.nlm.nih.gov/books/NBK470328/.
Griffin, Daniel O., et al. “Pulmonary Embolism and Increased Levels of D-Dimer in Patients with Coronavirus Disease.” Emerging Infectious Diseases, vol. 26, no. 8, Aug. 2020, pp. 1941–43. PubMed, https://doi.org/10.3201/eid2608.201477.
Jarvis, Kirsten Brunsvig, et al. “Candidate Single Nucleotide Polymorphisms and Thromboembolism in Acute Lymphoblastic Leukemia – A NOPHO ALL2008 Study.” Thrombosis Research, vol. 184, Dec. 2019, pp. 92–98. PubMed, https://doi.org/10.1016/j.thromres.2019.11.002.
Jiang, Lilong, et al. “Discovery of Glycyrrhetinic Acid as an Orally Active, Direct Inhibitor of Blood Coagulation Factor Xa.” Thrombosis Research, vol. 133, no. 3, Mar. 2014, pp. 501–06. PubMed, https://doi.org/10.1016/j.thromres.2013.12.025.
Khatami, Mehri, et al. “Common Rs5918 (PlA1/A2) Polymorphism in the ITGB3 Gene and Risk of Coronary Artery Disease.” Archives of Medical Sciences. Atherosclerotic Diseases, vol. 1, no. 1, Apr. 2016, pp. e9–15. PubMed Central, https://doi.org/10.5114/amsad.2016.59587.
Kim, Kyung-Min, et al. “Novel Factor Xa Inhibitor, Maslinic Acid, with Antiplatelet Aggregation Activity.” Journal of Cellular Physiology, vol. 235, no. 12, Dec. 2020, pp. 9445–56. PubMed, https://doi.org/10.1002/jcp.29749.
Komsa-Penkova, Regina, et al. “Rs5918ITGB3 Polymorphism, Smoking, and BMI as Risk Factors for Early Onset and Recurrence of DVT in Young Women.” Clinical and Applied Thrombosis/Hemostasis: Official Journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis, vol. 23, no. 6, Sept. 2017, pp. 585–95. PubMed, https://doi.org/10.1177/1076029615624778.
LaPelusa, Andrew, and Heeransh D. Dave. “Physiology, Hemostasis.” StatPearls, StatPearls Publishing, 2022. PubMed, http://www.ncbi.nlm.nih.gov/books/NBK545263/.
Lodigiani, Corrado, et al. “Venous and Arterial Thromboembolic Complications in COVID-19 Patients Admitted to an Academic Hospital in Milan, Italy.” Thrombosis Research, vol. 191, July 2020, pp. 9–14. PubMed, https://doi.org/10.1016/j.thromres.2020.04.024.
Melchinger, Hannah, et al. “Role of Platelet Mitochondria: Life in a Nucleus-Free Zone.” Frontiers in Cardiovascular Medicine, vol. 6, Oct. 2019, p. 153. PubMed Central, https://doi.org/10.3389/fcvm.2019.00153.
—. “Role of Platelet Mitochondria: Life in a Nucleus-Free Zone.” Frontiers in Cardiovascular Medicine, vol. 6, Oct. 2019, p. 153. PubMed Central, https://doi.org/10.3389/fcvm.2019.00153.
Mikkelsson, Jussi, et al. “Glycoprotein IIIa PlA1/A2 Polymorphism and Sudden Cardiac Death.” Journal of the American College of Cardiology, vol. 36, no. 4, Oct. 2000, pp. 1317–23. jacc.org (Atypon), https://doi.org/10.1016/S0735-1097(00)00871-8.
Motovska, Zuzana, et al. “Platelet Glycoprotein GP VI 13254C Allele Is an Independent Risk Factor of Premature Myocardial Infarction.” Thrombosis Research, vol. 125, no. 2, Feb. 2010, pp. e61-64. PubMed, https://doi.org/10.1016/j.thromres.2009.09.002.
Mufti, Ahmad H., et al. “The Common VWF Single Nucleotide Variants c.2365A>G and c.2385T>C Modify VWF Biosynthesis and Clearance.” Blood Advances, vol. 2, no. 13, July 2018, pp. 1585–94. PubMed Central, https://doi.org/10.1182/bloodadvances.2017011643.
Naess, I. A., et al. “Incidence and Mortality of Venous Thrombosis: A Population-Based Study.” Journal of Thrombosis and Haemostasis: JTH, vol. 5, no. 4, Apr. 2007, pp. 692–99. PubMed, https://doi.org/10.1111/j.1538-7836.2007.02450.x.
Obi, Andrea T., et al. “Empirical Systemic Anticoagulation Is Associated with Decreased Venous Thromboembolism in Critically Ill Influenza A H1N1 Acute Respiratory Distress Syndrome Patients.” Journal of Vascular Surgery. Venous and Lymphatic Disorders, vol. 7, no. 3, May 2019, pp. 317–24. PubMed, https://doi.org/10.1016/j.jvsv.2018.08.010.
Oliver, Kendra H., et al. “Pro32Pro33 Mutations in the Integrin Β3 PSI Domain Result in ΑIIbβ3 Priming and Enhanced Adhesion: Reversal of the Hypercoagulability Phenotype by the Src Inhibitor SKI-606.” Molecular Pharmacology, vol. 85, no. 6, June 2014, pp. 921–31. PubMed, https://doi.org/10.1124/mol.114.091736.
Saeed, Anjum, et al. “To Determine the Frequency of Factor V Leiden in Cases of Deep Vein Thrombosis and Healthy Controls.” Pakistan Journal of Medical Sciences, vol. 31, no. 5, Oct. 2015, pp. 1219–22. PubMed, https://doi.org/10.12669/pjms.315.8088.
Simone, Benedetto, et al. “Risk of Venous Thromboembolism Associated with Single and Combined Effects of Factor V Leiden, Prothrombin 20210A and Methylenetethraydrofolate Reductase C677T: A Meta-Analysis Involving over 11,000 Cases and 21,000 Controls.” European Journal of Epidemiology, vol. 28, no. 8, Aug. 2013, pp. 621–47. PubMed, https://doi.org/10.1007/s10654-013-9825-8.
Skjeflo, E. W., et al. “Staphylococcus Aureus-Induced Complement Activation Promotes Tissue Factor-Mediated Coagulation.” Journal of Thrombosis and Haemostasis, vol. 16, no. 5, 2018, pp. 905–18. Wiley Online Library, https://doi.org/10.1111/jth.13979.
—. “Staphylococcus Aureus-Induced Complement Activation Promotes Tissue Factor-Mediated Coagulation.” Journal of Thrombosis and Haemostasis, vol. 16, no. 5, 2018, pp. 905–18. Wiley Online Library, https://doi.org/10.1111/jth.13979.
Smith, Nicholas L., et al. “Genetic Variation Associated with Plasma von Willebrand Factor Levels and the Risk of Incident Venous Thrombosis.” Blood, vol. 117, no. 22, June 2011, pp. 6007–11. PubMed Central, https://doi.org/10.1182/blood-2010-10-315473.
Suchon, Pierre, et al. “Common Risk Factors Add to Inherited Thrombophilia to Predict Venous Thromboembolism Risk in Families.” TH Open: Companion Journal to Thrombosis and Haemostasis, vol. 3, no. 1, Jan. 2019, pp. e28–35. PubMed Central, https://doi.org/10.1055/s-0039-1677807.
Swystun, Laura L., and David Lillicrap. “Genetic Regulation of Plasma von Willebrand Factor Levels in Health and Disease.” Journal of Thrombosis and Haemostasis : JTH, vol. 16, no. 12, Dec. 2018, pp. 2375–90. PubMed Central, https://doi.org/10.1111/jth.14304.
Szczeklik, Andrzej, et al. “Relationship between Bleeding Time, Aspirin and the PlA1/A2 Polymorphism of Platelet Glycoprotein IIIa.” British Journal of Haematology, vol. 110, no. 4, 2000, pp. 965–67. Wiley Online Library, https://doi.org/10.1046/j.1365-2141.2000.02267.x.
Tabeshpour, Jamshid, et al. “The Regulatory Role of Curcumin on Platelet Functions.” Journal of Cellular Biochemistry, vol. 119, no. 11, Nov. 2018, pp. 8713–22. PubMed, https://doi.org/10.1002/jcb.27192.
Tang, Weihong, et al. “Gene-Centric Approach Identifies New and Known Loci for FVIII Activity and VWF Antigen Levels in European Americans and African Americans.” American Journal of Hematology, vol. 90, no. 6, June 2015, pp. 534–40. PubMed Central, https://doi.org/10.1002/ajh.24005.
Undas, A., et al. “Pl(A2) Polymorphism of Beta(3) Integrins Is Associated with Enhanced Thrombin Generation and Impaired Antithrombotic Action of Aspirin at the Site of Microvascular Injury.” Circulation, vol. 104, no. 22, Nov. 2001, pp. 2666–72. PubMed, https://doi.org/10.1161/hc4701.099787.
Wei, Guangyu, et al. “Salidroside Inhibits Platelet Function and Thrombus Formation through AKT/GSK3β Signaling Pathway.” Aging, vol. 12, no. 9, Apr. 2020, pp. 8151–66. PubMed, https://doi.org/10.18632/aging.103131.
Xing, Changyang, et al. “Lung Ultrasound Findings in Patients with COVID-19 Pneumonia.” Critical Care, vol. 24, Apr. 2020, p. 174. PubMed Central, https://doi.org/10.1186/s13054-020-02876-9.