Key takeaways:
~ Uric acid is formed from the breakdown of purines.
~ Genetics and diet both play a role.
~ Uric acid levels are ~70% genetic.
~ Not everyone with high uric acid will have gout, but it can have other negative effects on health.
This article explains how uric acid is formed and why your genetic variants could increase your risk of gout.
Uric acid and gout:
Have you ever woken up to excruciating pain in your big toe or ankle? If so, you may be among the millions of people worldwide with gout. In fact, a study from 2008 showed that about 8.3 million Americans had gout. Men are affected at about 3 times the rate as women.[ref]
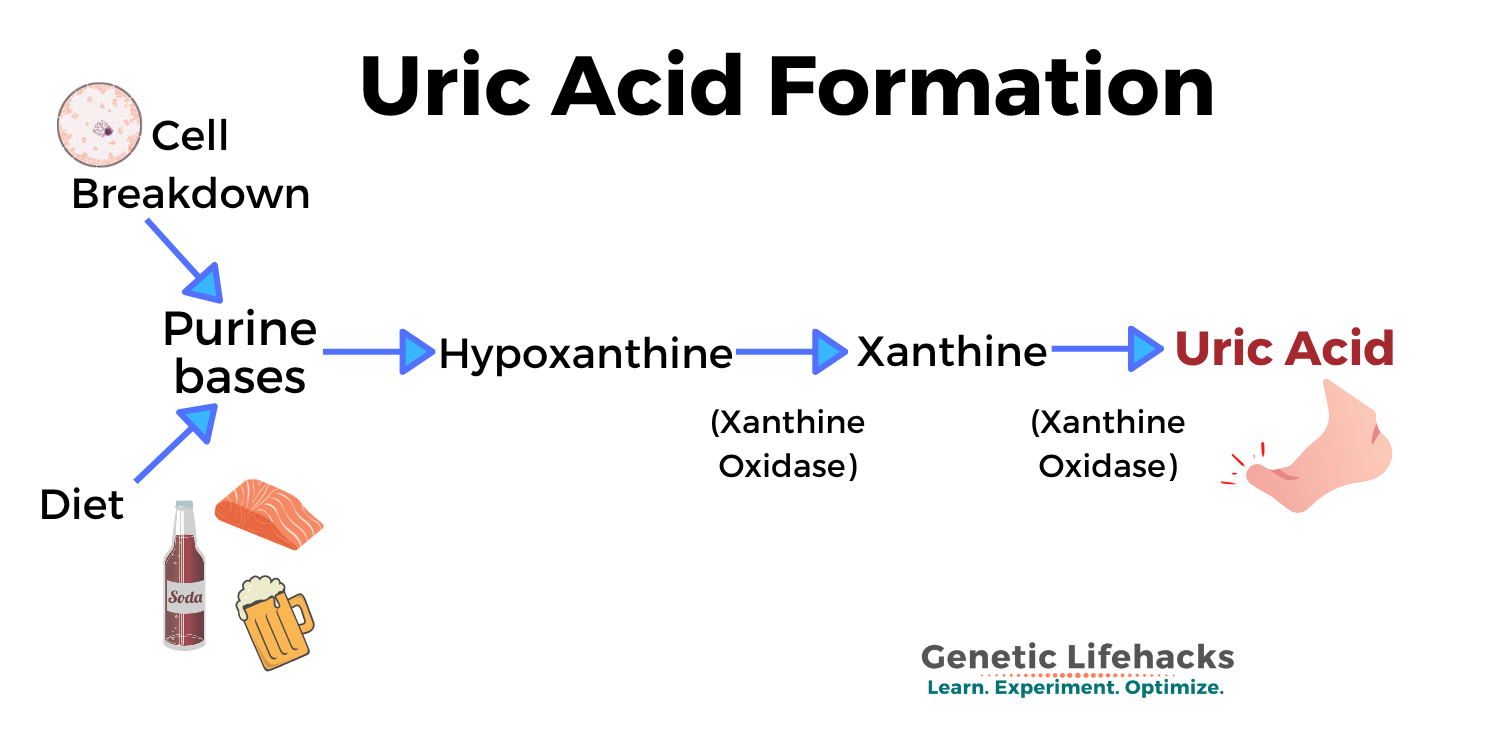
When your body breaks down purines, a type of biological molecule, the eventual byproduct of the process is uric acid. And just like it sounds like – uric acid is a normal part of the urine.
This biological process of breaking down purines and thus creating uric acid occurs for both purines created in the body and due to dietary factors. While it is easy to blame the dietary components (alcohol, fructose, meat, and seafood), the purines produced in the body are a big part of your uric acid levels.
Almost all other mammals go one step further when metabolizing purines, breaking down the uric acid into allantoin. Humans and higher primates don’t make the final enzyme to convert uric acid into allantoin. (Dalmations also have a genetic defect causing them to excrete uric acid instead of allantoin.) Researchers theorize that uric acid is important in humans and possibly takes the place of ascorbic acid (which humans and higher primates also don’t produce). Uric acid acts as a potent antioxidant in the serum, but can act as a pro-oxidant at certain levels and inside cells.[ref][ref]
Metabolism of purines:
Purines are a class of biological molecules that include the nucleotides adenine and guanine – the A’s and G’s in your DNA. You naturally have some cells in the body that are broken down each day. Cellular turnover is high in certain tissues (like the intestines and skin) and low in other tissues (like the brain) – but the net effect is that your body breaks down cellular components, including DNA and RNA, all the time.
Guanine and adenine are broken down through a multistep process that eventually creates xanthine, which is acted on by the enzyme xanthine oxidase to form uric acid. (This is important in how gout drugs work.)
What causes high uric acid?
The levels of uric acid in the body are primarily due to a person’s rate of turnover of cells as well as the rate of excretion and reabsorption in the kidneys.[ref]
In addition to someone’s natural rate of uric acid production and excretion, the following can influence uric acid levels:
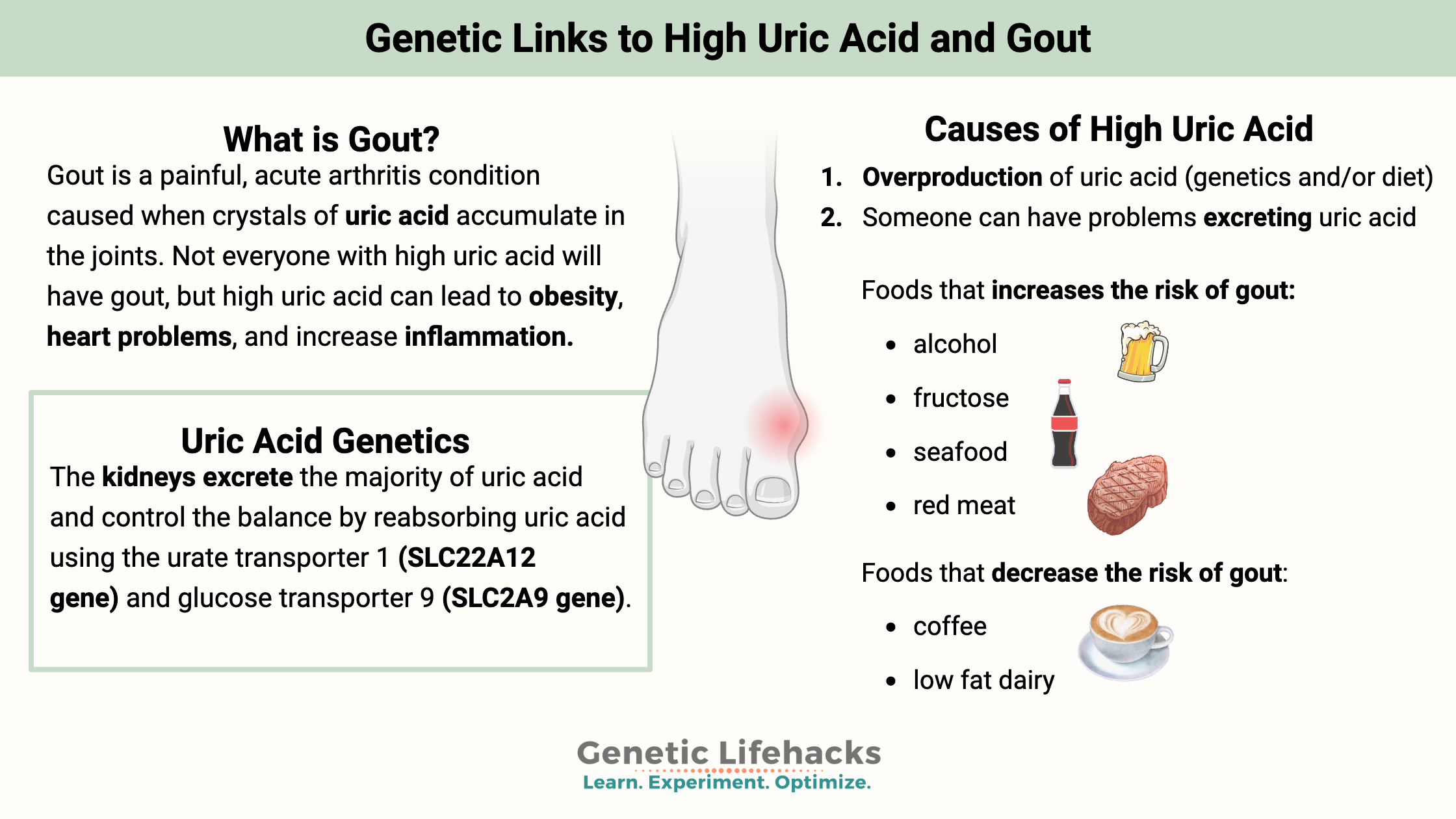
- Diet – high intake of fructose and purines (meat, seafood) increases uric acid[ref]
- Genetics – uric acid levels are considered about 70% heritable (due to genetics)[ref]
- Kidney disease doubles the risk of gout[ref]
- Fasting and rapid weight loss increase uric acid (temporarily)
- Type-2 diabetes[ref]
- Medications
- Blood cancers
Medications: The thiazide class of diuretics used for high blood pressure significantly increases the risk of high uric acid in men.[ref]
Overproduction vs. Under Excretion:
There are two ways of looking at high uric acid levels:
- Someone can create too much uric acid (genetics and/or diet)
- Someone can have problems excreting uric acid
The third phenotype here is a combination of both creating too much uric acid along with not excreting enough.[ref]
Figuring out which genetic variants you carry that impact these pathways may help you determine the best method of decreasing uric acid levels – personalized for you.
Kidney Excretion of Uric Acid
The kidneys excrete the majority of uric acid and are the regulators of uric acid levels in the body. They control the balance by reabsorbing uric acid using the urate transporter 1 (SLC22A12 gene) and glucose transporter 9 (SLC2A9 gene).[ref]
How high is too high? Serum uric acid level:
Lab ranges may vary a bit on uric acid regarding what is considered normal vs. high. Basically, there comes a point when crystals will form when the concentration of uric acid gets too high.
The normal reference range for uric acid is:
- 1.5 – 6.0 mg/dL for adult women
- 2.5 to 7.0 mg/dL for adult men
In blood, uric acid crystals start to form at 6.8 mg/dL.[ref]
Normally, about 70% of uric acid is disposed of each day via the kidneys, with the rest being excreted through feces.[ref] High uric acid levels are mainly driven by inefficient renal excretion.[ref]
What exactly is gout?
Gout is a painful, acute arthritis condition caused when crystals of uric acid accumulate in the joints.
Uric acid crystals can also form in the capillaries and skin, but for most people, the first onset of gout is heralded by pain and swelling in the big toe.
Genetics plays a significant role in gout, with heritability estimated at 73% (which is high!).[ref]
Gout occurs with high uric acid levels, but the exact level at which gout occurs varies a lot from person to person.
Getting more detailed:
High uric acid levels for extended periods cause the growth of monosodium urate crystals in and around the joints. These crystals are initially asymptomatic, but they can be seen on ultrasound. The acute episodes of pain are thought to be caused by crystals moving from the cartilage surface into the space in the joint. Ouch! If uric acid remains elevated, the crystals keep forming and periodically move into the joint space – leading to chronic gout, which can damage the joint.[ref]
Another component of gout pain is increased inflammation due to the monosodium urate crystals.
Gout treatments:
Xanthine oxidase inhibitors, such as allopurinol and febuxostat, lower urate levels and help prevent gout attacks.[ref]
Lifestyle interventions, such as dietary changes, have also been shown to be effective in lowering gout risk. See the Lifehacks section below.
Gout is not a new health problem, although it is increasing. First written about by the ancient Egyptians and by Hippocrates in the 1800s, gout was treated with lithium salts since lithium binds to urate to make it more soluble.
Lithium was (and still is) commonly used in studies when giving test subjects uric acid. In fact, this is how lithium came to be used for bipolar disorder. A psychiatrist in 1949 started treating bipolar patients with lithium carbonate after noticing lab animals were calmer after being treated with lithium plus uric acid.[ref]
High uric acid level, but without gout:
The majority of people with high uric acid levels actually do not have gout. A study based on data from 2008 found that only 4% of US adults have gout, while over 21% of adults have high uric acid levels. This study defined hyperuricemia as serum urate levels of >5.7 mg/dl for women and >7 mg/dl for men.
Many association studies show that people with high uric acid are also more likely to have ‘co-morbidities’ or other health conditions. Questions remain, though, as to whether there is causation involved – for example: do high uric acid levels cause high triglycerides, or do high triglycerides cause high uric acid?[ref] Is high uric acid just a byproduct (perhaps due to diet), or is it playing a role in causing chronic disease?
Obesity:
Obesity is one co-morbidity occurring at a higher rate in people with higher uric acid. Some studies indicate obesity causes high serum uric acid.[ref] Other studies indicate that high uric acid levels cause an increase in obesity.[ref]
In fact, some researchers theorize that humans found it advantageous to have higher uric acid levels because it promoted fat accumulation, which was helpful for survival throughout history.[ref]
Interestingly, there seems to be a U-shaped curve for uric acid levels and BMI, with the lowest uric acid found in women with a BMI of 21.3 and men with a BMI of 19.1.[ref]
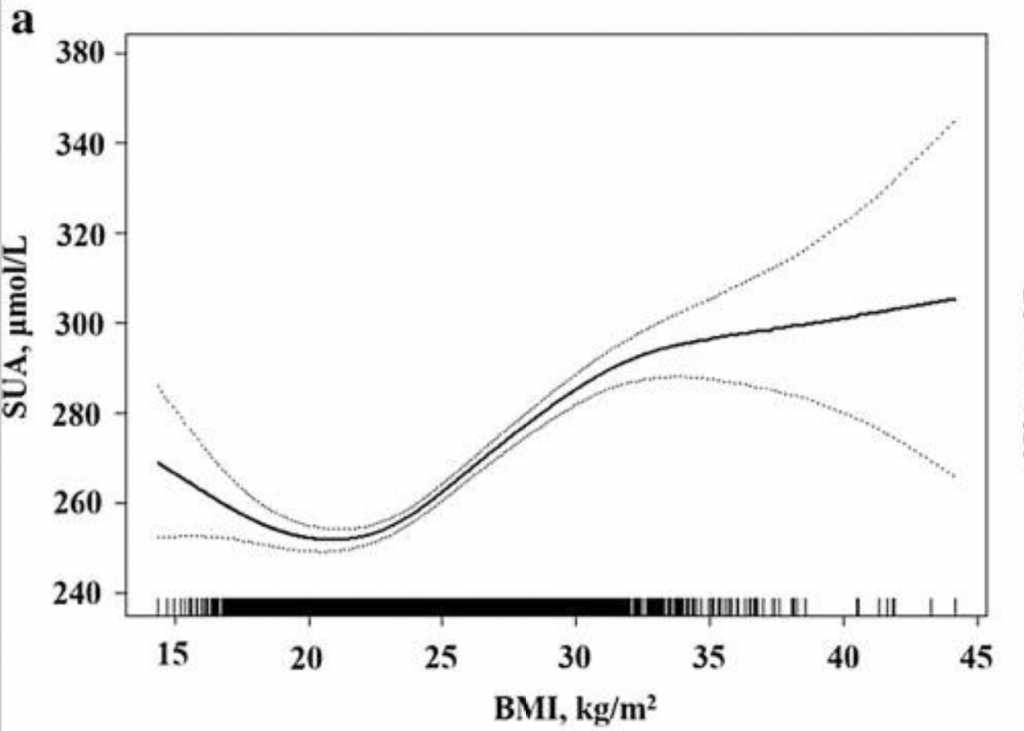
While the complete picture isn’t clear here, obesity combined with higher uric acid levels is also associated with a greater risk of high blood pressure than either condition separately.[ref]
Heart problems:
High uric acid levels are linked with a greater risk of heart problems and cardiovascular mortality. A study showed cardiac patients with uric acid levels >8mg/dL had a more than 4-fold increase in the 2-year risk of cardiac mortality. The trend on this seemed to be fairly linear, with people with uric acid >8 at the highest risk for death; uric acid between 7 and 8 still at elevated risk; to people with uric acid less than 6 being at the lowest risk for cardiac mortality.[ref] Again – I’m not sure causality is proven here as to whether uric acid increases heart problems or whether heart disease (and heart cell death) increases uric acid.
Inflammation:
A really interesting study looked at the effects of giving uric acid to healthy people with normal uric acid levels.
Giving people uric acid, of course, raised their uric acid levels. Researchers caused increased uric acid and then had the study participants eat a fast food meal containing 900 calories and 50 grams of fat. The researchers then tested their inflammatory markers at 2-hour intervals. They compared the markers with the same meal given a couple of days later – but with lowered uric acid levels. The results showed high uric acid, combined with the high-fat fast-food meal, causes a significant increase in the inflammatory cytokine IL-6.[ref]
Low uric acid: What does it mean?
Like most things in the body, there seems to be a sweet spot for uric acid – neither too high nor too low. Uric acid does act as a potent antioxidant in the serum, and it is thought to contribute about 60% to the plasma antioxidant capacity in the body.[ref]
Studies show that uric acid levels are lower in people with Parkinson’s disease and that high uric acid levels are protective against Parkinson’s.[ref][ref] There is also a link between quicker progression in Alzheimer’s with low uric acid. And people with higher uric acid levels are at a reduced risk of Alzheimer’s.[ref]
Gout Genotype Report:
Lifehacks:
Testing your uric acid levels:
Your doctor can order a uric acid blood test if you suspect you have gout.
If, for some reason, you can’t order the test through your doctor, you can order blood tests yourself online in the US through Ulta Lab tests and other online lab test ordering companies. (UltaLabs Tests price – $18) It is also a good option if you are trying different lifestyle interventions and want to track the results.
Fructose consumption increases uric acid:
It is always interesting to see how researchers create an animal model of the human condition. Researchers simply gave rats 10% fructose in their water to create high uric acid levels.[ref]
In humans, there is a strong relationship between the intake of fructose and uric acid levels. When fructose is metabolized in the liver, it is broken down through a multi-step process. When too much fructose is consumed at once (e.g., drinking a large soda containing high-fructose corn syrup), one of the components of the metabolism process can build up in the liver. It stimulates purine degradation, which produces uric acid.[ref]
Not all studies agree on the role fructose plays in uric acid –including a large meta-analysis of controlled trials.[ref]
The key may be the amount of fructose consumed at one time, with larger amounts overwhelming the liver.[ref]
Moderating fructose consumption and not consuming larger amounts at one time may be key here.
Specific Lifehacks for Variants:
ABCG2 variant carriers:
Related Articles and Topics:
Oxalates, Kidney Stones, & Joint Pain: Genetic Reasons to Avoid Oxalates
Familial Mediterranean Fever: Mimics fibromyalgia, arthritis, inflammation
References:
Abu Bakar, Fazleen I., et al. “Anti-Gout Potential of Malaysian Medicinal Plants.” Frontiers in Pharmacology, vol. 9, Mar. 2018, p. 261. PubMed Central, https://doi.org/10.3389/fphar.2018.00261.
Brackman, Deanna J., et al. “Genome-Wide Association and Functional Studies Reveal Novel Pharmacological Mechanisms for Allopurinol.” Clinical Pharmacology and Therapeutics, vol. 106, no. 3, Sept. 2019, pp. 623–31. PubMed Central, https://doi.org/10.1002/cpt.1439.
Caliceti, Cristiana, et al. “Fructose Intake, Serum Uric Acid, and Cardiometabolic Disorders: A Critical Review.” Nutrients, vol. 9, no. 4, Apr. 2017, p. 395. PubMed Central, https://doi.org/10.3390/nu9040395.
Dehghan, Abbas, et al. “Association of Three Genetic Loci with Uric Acid Concentration and Risk of Gout: A Genome-Wide Association Study.” Lancet, vol. 372, no. 9654, Dec. 2008, pp. 1953–61. PubMed Central, https://doi.org/10.1016/S0140-6736(08)61343-4.
Duong, Nguyen Thuy, et al. “Polymorphisms of ABCG2 and SLC22A12 Genes Associated with Gout Risk in Vietnamese Population.” Medicina (Kaunas, Lithuania), vol. 55, no. 1, Jan. 2019, p. E8. PubMed, https://doi.org/10.3390/medicina55010008.
Higashino, Toshihide, et al. “Multiple Common and Rare Variants of ABCG2 Cause Gout.” RMD Open, vol. 3, no. 2, 2017, p. e000464. PubMed, https://doi.org/10.1136/rmdopen-2017-000464.
Ichida, Kimiyoshi, et al. “Decreased Extra-Renal Urate Excretion Is a Common Cause of Hyperuricemia.” Nature Communications, vol. 3, Apr. 2012, p. 764. PubMed Central, https://doi.org/10.1038/ncomms1756.
Jin, Ming, et al. “Uric Acid, Hyperuricemia and Vascular Diseases.” Frontiers in Bioscience : A Journal and Virtual Library, vol. 17, Jan. 2012, pp. 656–69. PubMed Central, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3247913/.
Khunweeraphong, Narakorn, et al. “The ABCG2 Multidrug Transporter Is a Pump Gated by a Valve and an Extracellular Lid.” Nature Communications, vol. 10, Nov. 2019, p. 5433. PubMed Central, https://doi.org/10.1038/s41467-019-13302-2.
Kobayashi, Nobuaki, et al. “Impact of Accumulated Serum Uric Acid on Coronary Culprit Lesion Morphology Determined by Optical Coherence Tomography and Cardiac Outcomes in Patients with Acute Coronary Syndrome.” Cardiology, vol. 141, no. 4, 2018, pp. 190–98. PubMed, https://doi.org/10.1159/000496053.
Li, Rongrong, et al. “Dietary Factors and Risk of Gout and Hyperuricemia: A Meta-Analysis and Systematic Review.” Asia Pacific Journal of Clinical Nutrition, vol. 27, no. 6, 2018, pp. 1344–56. PubMed, https://doi.org/10.6133/apjcn.201811_27(6).0022.
Li, Zhiqiang, et al. “Replication of Gout/Urate Concentrations GWAS Susceptibility Loci Associated with Gout in a Han Chinese Population.” Scientific Reports, vol. 7, June 2017, p. 4094. PubMed Central, https://doi.org/10.1038/s41598-017-04127-4.
Liu, Jia, et al. “ABCG2 Rs2231142 Variant in Hyperuricemia Is Modified by SLC2A9 and SLC22A12 Polymorphisms and Cardiovascular Risk Factors in an Elderly Community-Dwelling Population.” BMC Medical Genetics, vol. 21, no. 1, Mar. 2020, p. 54. PubMed, https://doi.org/10.1186/s12881-020-0987-4.
Lyngdoh, Tanica, et al. “Serum Uric Acid and Adiposity: Deciphering Causality Using a Bidirectional Mendelian Randomization Approach.” PloS One, vol. 7, no. 6, 2012, p. e39321. PubMed, https://doi.org/10.1371/journal.pone.0039321.
Nigam, Sanjay K., and Vibha Bhatnagar. “The Systems Biology of Uric Acid Transporters: The Role of Remote Sensing and Signaling.” Current Opinion in Nephrology and Hypertension, vol. 27, no. 4, July 2018, pp. 305–13. PubMed Central, https://doi.org/10.1097/MNH.0000000000000427.
Perez-Ruiz, F., et al. “Renal Underexcretion of Uric Acid Is Present in Patients with Apparent High Urinary Uric Acid Output.” Arthritis and Rheumatism, vol. 47, no. 6, Dec. 2002, pp. 610–13. PubMed, https://doi.org/10.1002/art.10792.
Perez-Ruiz, Fernando, et al. “A Review of Uric Acid, Crystal Deposition Disease, and Gout.” Advances in Therapy, vol. 32, no. 1, 2015, pp. 31–41. PubMed Central, https://doi.org/10.1007/s12325-014-0175-z.
Rasheed, Humaira, et al. “Mendelian Randomization Provides No Evidence for a Causal Role of Serum Urate in Increasing Serum Triglyceride Levels.” Circulation. Cardiovascular Genetics, vol. 7, no. 6, Dec. 2014, pp. 830–37. PubMed, https://doi.org/10.1161/CIRCGENETICS.114.000556.
Roughley, Matthew J., et al. “Gout and Risk of Chronic Kidney Disease and Nephrolithiasis: Meta-Analysis of Observational Studies.” Arthritis Research & Therapy, vol. 17, Apr. 2015, p. 90. PubMed, https://doi.org/10.1186/s13075-015-0610-9.
Roumeliotis, Stefanos, et al. “Dietary Antioxidant Supplements and Uric Acid in Chronic Kidney Disease: A Review.” Nutrients, vol. 11, no. 8, Aug. 2019, p. 1911. PubMed Central, https://doi.org/10.3390/nu11081911.
Schlesinger, Ilana, and Naomi Schlesinger. “Uric Acid in Parkinson’s Disease.” Movement Disorders: Official Journal of the Movement Disorder Society, vol. 23, no. 12, Sept. 2008, pp. 1653–57. PubMed, https://doi.org/10.1002/mds.22139.
Singh, Jasvinder A., et al. “Comparative Effectiveness of Urate Lowering with Febuxostat versus Allopurinol in Gout: Analyses from Large U.S. Managed Care Cohort.” Arthritis Research & Therapy, vol. 17, no. 1, 2015, p. 120. PubMed Central, https://doi.org/10.1186/s13075-015-0624-3.
Stark, Klaus, et al. “Association of Common Polymorphisms in GLUT9 Gene with Gout but Not with Coronary Artery Disease in a Large Case-Control Study.” PLoS ONE, vol. 3, no. 4, Apr. 2008, p. e1948. PubMed Central, https://doi.org/10.1371/journal.pone.0001948.
Tana, Claudio, et al. “Uric Acid and Cognitive Function in Older Individuals.” Nutrients, vol. 10, no. 8, July 2018, p. 975. PubMed Central, https://doi.org/10.3390/nu10080975.
Tanaka, Toshiko, et al. “A Double Blind Placebo Controlled Randomized Trial of the Effect of Acute Uric Acid Changes on Inflammatory Markers in Humans: A Pilot Study.” PLoS ONE, vol. 12, no. 8, Aug. 2017, p. e0181100. PubMed Central, https://doi.org/10.1371/journal.pone.0181100.
Tian, Simiao, et al. “Does Obesity Modify the Epidemiological Association between Hyperuricemia and the Prevalence of Hypertension among Northern Chinese Community-Dwelling People? A Chinese Population-Based Study.” BMJ Open, vol. 9, no. 11, Nov. 2019, p. e031803. PubMed, https://doi.org/10.1136/bmjopen-2019-031803.
Tondo, Leonardo, et al. “Clinical Use of Lithium Salts: Guide for Users and Prescribers.” International Journal of Bipolar Disorders, vol. 7, July 2019, p. 16. PubMed Central, https://doi.org/10.1186/s40345-019-0151-2.
Wang, Yu, et al. “Cichorium Intybus L. Promotes Intestinal Uric Acid Excretion by Modulating ABCG2 in Experimental Hyperuricemia.” Nutrition & Metabolism, vol. 14, June 2017, p. 38. PubMed Central, https://doi.org/10.1186/s12986-017-0190-6.
Zemunik, Tatijana, et al. “Genome-Wide Association Study of Biochemical Traits in Korcula Island, Croatia.” Croatian Medical Journal, vol. 50, no. 1, Feb. 2009, pp. 23–33. PubMed, https://doi.org/10.3325/cmj.2009.50.23.
Zhao, Rui, et al. “Gout and Risk of Diabetes Mellitus: Meta-Analysis of Observational Studies.” Psychology, Health & Medicine, vol. 25, no. 8, Sept. 2020, pp. 917–30. PubMed, https://doi.org/10.1080/13548506.2019.1707241.
Zheng, Rongjiong, et al. “Serum Uric Acid Levels and the Risk of Obesity: A Longitudinal Population-Based Epidemiological Study.” Clinical Laboratory, vol. 63, no. 10, Oct. 2017, pp. 1581–87. PubMed, https://doi.org/10.7754/Clin.Lab.2017.170311.
Zhong, Ling-Ling, et al. “Level of Uric Acid and Uric Acid/Creatinine Ratios in Correlation with Stage of Parkinson Disease.” Medicine, vol. 97, no. 26, June 2018, p. e10967. PubMed, https://doi.org/10.1097/MD.0000000000010967.
Zhou, Hui, et al. “Nonlinear Relationship between Serum Uric Acid and Body Mass Index: A Cross-Sectional Study of a General Population in Coastal China.” Journal of Translational Medicine, vol. 17, Nov. 2019, p. 389. PubMed Central, https://doi.org/10.1186/s12967-019-02142-9.
Zhou, Zhaowei, et al. “Common Variants in the SLC28A2 Gene Are Associated with Serum Uric Acid Level and Hyperuricemia and Gout in Han Chinese.” Hereditas, vol. 156, Jan. 2019, p. 4. PubMed Central, https://doi.org/10.1186/s41065-018-0078-0.
Zhu, Yanyan, et al. “Prevalence of Gout and Hyperuricemia in the US General Population: The National Health and Nutrition Examination Survey 2007-2008.” Arthritis and Rheumatism, vol. 63, no. 10, Oct. 2011, pp. 3136–41. PubMed, https://doi.org/10.1002/art.30520.