Key Takeaways:
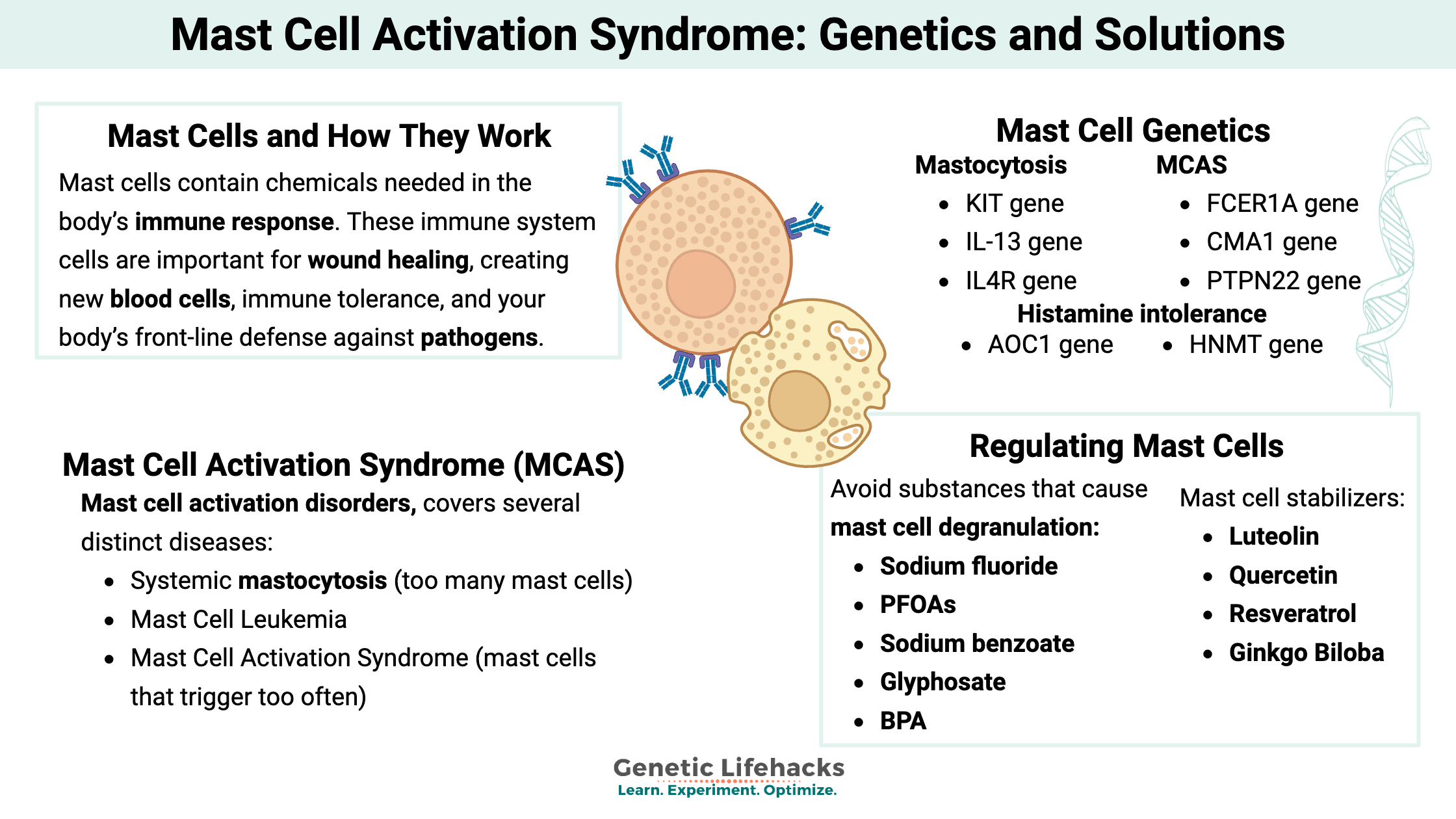
~Mast cells are essential immune cells, but can cause problems if overactive.
~ Mast Cell Activation Syndrome (MCAS) involves mast cells that are activated easily.
~Symptoms of MCAS can include abdominal pain, nausea, itching, flushing, hives, headaches, heart palpitations, anxiety, brain fog, and anaphylaxis.
~ Mast cell disorders overlap with many other conditions, including autoimmune and connective tissue diseases.
~ Genetic variants interact with susceptibility to MCAS in multiple ways.
This article explains how mast cells work and what happens when they are overactive. We will dive into some genetic factors and explore possible solutions for MCAS.
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Consider joining today.
What are mast cells?
Mast cells, a type of granulocyte, contain chemicals needed in the body’s immune response. These immune system cells are important for wound healing, creating new blood cells, immune tolerance, and your body’s front-line defense against pathogens.
As one study summed up, mast cells are the ‘jack of all trades’ immune cells.
Mast cells are a part of our innate immune system and are vital to our body’s response to invaders. All good… until things go haywire.
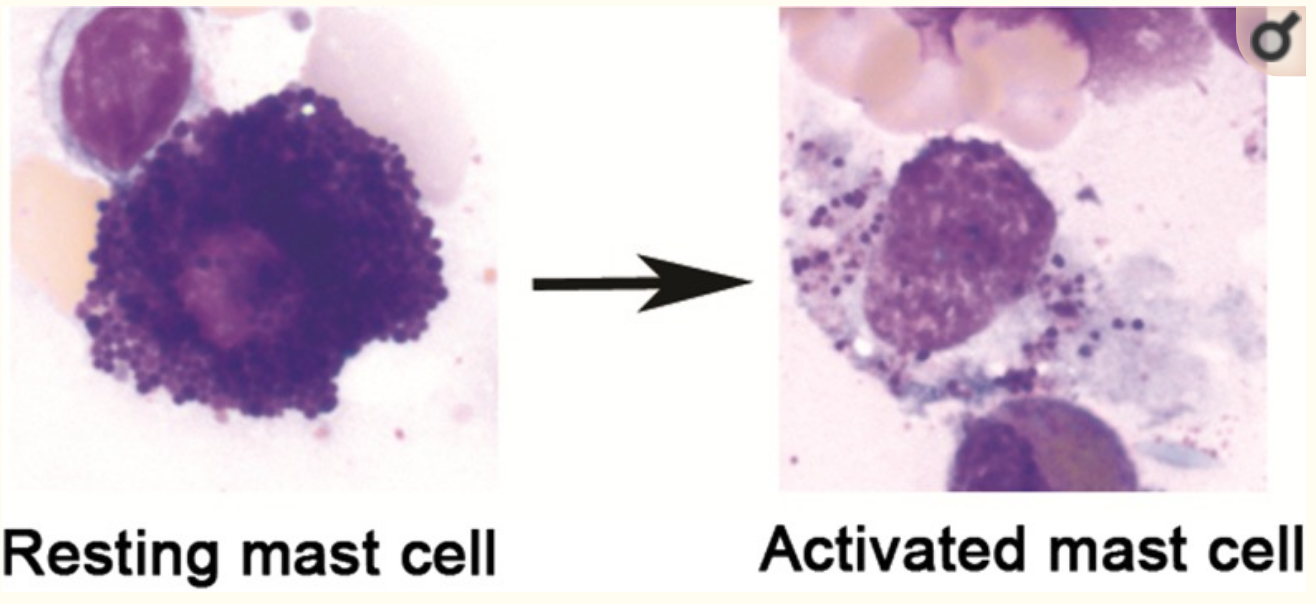
When mast cells trigger too easily, they degranulate and release a payload of histamine, heparin, prostaglandins, tryptase, and cytokines into the body too frequently. It causes a variety of complications with seemingly disjointed symptoms.
The classic function of mast cells in the immune system:
This section will explain how mast cells usually function within the immune system (skip ahead if you already know all this…)
Mast cells are resident immune cells, meaning they aren’t usually in the bloodstream but instead reside in tissue, such as connective tissue. For example, mast cells reside in the areas next to epithelial cells (skin cells, the lining of the intestines, and surface cells in the lungs) or near endothelial cells, which make up blood vessels.
Mast cells are classified as granulocytes, which are cells that contain granules with several types of molecules within them. Kind of like little water balloons, mast cells can degranulate quickly — releasing various inflammatory molecules into the surrounding tissue.
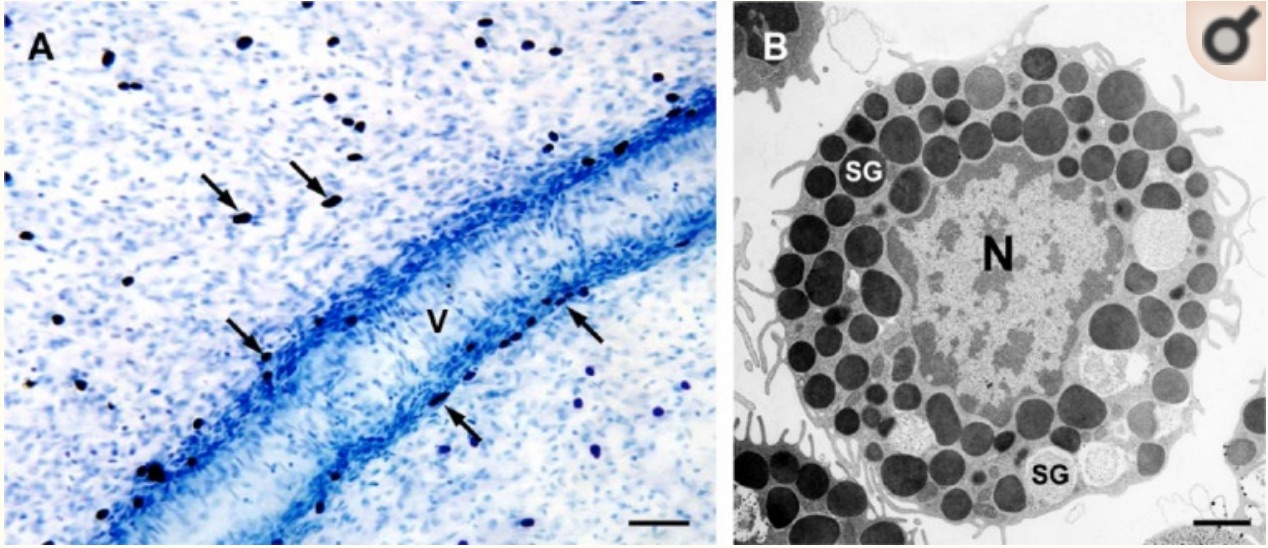
IgE Antibodies and Immune Reactions:
As immune cells, mast cells respond to pathogens, such as viruses, parasites, and bacteria.
Here’s how it works:[ref]
FcɛRI receptors are on the surface of mast cells. They bind to IgE antibodies created by Th2 cells and trigger B-cells to differentiate and create IgE. Usually, this happens due to a pathogen or parasite.
IgE binds to the FcɛRI receptor on mast cells. It primes the mast cell for activation as soon as the other half of the IgE binds to an antigen (on a pathogen/parasite/allergen). Called cross-linking, the FcɛRI binds to IgE, which then binds to an antigen.
Traditional mast cell activation (antigen binding) causes both:
an immediate reaction
– and –
a delayed reaction
Immediate reaction:
The immediate reaction happens quickly, within minutes, causing the mast cell to degranulate and release its chemical-filled granules into the surrounding area.
- Histamine is released from granules in mast cells. Histamine is often thought of with allergy-type symptoms, but it is also toxic to pathogens. In a nutshell, when histamine binds to histamine receptors on a cell, it causes an inflammatory immune response. For example, when histamine binds to a receptor on endothelial (blood vessel) cells, it causes vascular permeability, resulting in swelling and inflammation. When histamine binds to histamine receptors on smooth muscle cells, it can cause the cells to contract. In the gastrointestinal tract, this causes vomiting and diarrhea to get rid of the pathogen. By contracting the muscles around the lungs, histamine causes coughing, sneezing, and wheezing to eliminate the pathogen.
- Proteases, enzymes that break down proteins, are also released during degranulation. The mast cells mainly reside in connective tissue, in which the extracellular matrix is made of proteins that hold the tissue together. Breaking down that extracellular matrix using proteases allows fluid to come in and flush out the body.
- TNF-alpha is also found in mast cells. It is an inflammatory cytokine binding to receptors on the surface of endothelial (blood vessel) cells, making endothelial cells turn on adhesion molecules and recruit more immune cells.
Delayed reaction:
There is also a later response that happens hours after the initial trigger of mast cell degranulation.
- IL-4 and Th2 cell production is increased after mast cell degranulation. Mast cells also produce chemokines such as CCL3, which signal monocytes and neutrophils to the area. GM-CSF, made in mast cells, targets bone marrow stem cells, telling them to differentiate into basophils and monocytes. Basically, this calls up reserve troops for the immune response to continue fighting off the pathogens.
- Prostaglandins and leukotrienes are also synthesized by mast cells. These induce inflammation. Leukotrienes are 100 times more potent than histamine but have similar actions. Prostaglandins cause inflammation via vasodilation, vascular permeability, and more immune cells to become attracted to fight the infection. Prostaglandin also induces pain via binding to sensory neurons. Prostaglandins are synthesized from arachidonic acid using cyclooxygenase (COX), and you can inhibit prostaglandin response via aspirin or NSAIDs (e.g., ibuprofen).
Overall, mast cells have 50 – 200 granules in the cytoplasm of the cell, storing histamine, heparin, tryptase, cytokines, etc.[ref] The image below shows mast cell (stained purple) degranulation under a microscope.[ref]
Related Article: Mast cells, spike protein, heart rhythm (article explaining how heart rhythm regulation relates to mast cells, along with the research on how the spike protein activates mast cells)
Allergies: Activating mast cells by Type 1 hypersensitivity
In addition to reacting to antigens on pathogens, such as bacteria, the IgE can also be primed to link with antigens on proteins that are not pathogens, referred to as allergens. Common allergens include pollen, cats, dust mites, nuts, dairy, and fish. The entire ‘becoming allergic to something’ process is quite involved. The common protein must be taken in by macrophages or dendritic cells to start the process, causing an antigen presentation on MHC II molecules. It tends to happen with inherited MHC-II types – certain HLA types. When these antigens are presented, they could bind to a CD4 receptor that activates the CD4 T cells. It could prime the T-cell to become a TH2 helper T-cell if IL-4 is present. B-cells do something here also… if they also have the allergen protein that they have phagocytosed and presented, then the B-cell binds to the Th2 cell and creates IgE. The IgE binds to the mast cells.
If the allergen returns, it binds to the IgE on mast cells and causes degranulation. What happens in the allergic response depends on where the mast cells activate (gut, airway, skin, etc.). For example, inhaled airborne allergens bind to IgE on the mast cells and trigger a response. The response in the nose is allergic rhinitis (swelling, fluid, sneezing), while activation in the lungs causes allergic asthma, fluid and mucus deep in the lungs, bronchial constriction, and coughing.
Related article: Food allergies, grass allergies
Viruses and mast cells:
While textbook information on mast cell activation usually mentions parasites (worms), bacteria, and allergens, more recent research shows that viruses also activate mast cells. A 2015 paper shows that the influenza A virus directly activates mast cells, causing the release of cytokines and contributing to the excessive inflammatory response in the lungs.[ref] Animal studies show that this pattern of mast cell activation and excessive response holds true for several flu strains. Additionally, mast cell inhibitors dramatically reduced lung lesions and mortality rates from the flu.[ref]
Other substances that activate mast cells:
IgE activation is just one way a mast cell activates during its normal pathogen-fighting function.
The following are known triggers of mast cell degranulation (in addition to IgG activation via pathogen or allergen):[ref]
- Acetylcholine
- Complement fragments (C3α, C4α, C5α)
- Various drugs, environmental toxins
- Peptides (including endorphin, leptin, PTH, and more)
- Physical conditions (cold, heat, pressure, stress, and vibration).
Additionally, mast cell activation can occur by IgG-antigen complexes, pathogen-associated molecular patterns (PAMPS), cell-cell contact, and hormones.[ref]
Mast cell release exosomes:
In addition to degranulation when stimulated, mast cells also communicate with other cells by secreting mediators and by releasing exosomes. In contrast to degranulation, exosome release is controlled and sustained. The proteins, lipids, and microRNAs carried by the exosomes are wrapped in a membrane that can then fuse with other cells. These released exosomes can then modulate immune response in other cells through a payload of inflammatory cytokines, like TNF-alpha, or through microRNAs that tamp down inflammation.[ref]
Mast cell release of exosomes has been shown to increase the formation of collagen in fibroblasts, which can lead to fibrosis (including pulmonary fibrosis). Mast cell exosomes and the microRNAs carried are also involved in pre-eclampsia in pregnancy. In the clotting pathways, mast cell exosomes interact with plasminogen activator inhibitor-1 (PAI-1). [ref]
Recap and key points about mast cells
- An essential part of the immune system, mast cells reside near all of the body’s external surfaces (skin, intestine, lungs) as well as on the outside of blood vessels, in connective joint tissue, and near peripheral nerves.[ref]
- The mast cell precursors form from bone marrow stem cells and travel through the blood to different tissues, where they will mature and reside.[ref]
- Mast cells in different tissues act differently — secreting different chemicals when activated. For example, some release only tryptase, and others only chymase (both along with histamine, IL-6, TNF-alpha, prostaglandins, etc.).[ref]
- Allergens and pathogens cross-link with IgE and activate mast cells. It causes an immediate release of almost all the granules (containing serotonin, histamine, chemokines, cytokines, enzymes, heparin, and prostaglandins), which trigger itching, swelling, mucous, and anaphylaxis.[ref]
- A delayed response by mast cells comes from the formation of other mediators, such as prostaglandin D2.
- Mast cells can also activate through receptors other than IgE-mediated and will only release certain, specific granules (e.g., just histamine).
- Mast cells can also become overly sensitized (more below) and degranulate due to substances produced within the body or physical conditions.
When mast cells misbehave…
We need mast cells for an immune response to fight bacteria when we get a cut on our fingers. But overactive mast cells – either too many are made or are triggered too easily – can cause a cascade of different symptoms. Research on the different mast cell disorders dates back decades. New research has led to greater understanding and evolving definitions and diagnoses. (In other words, forgive me if I didn’t define something in a way more familiar to you.)
Below is a brief overview of research on mast cell activation disorders, which is an umbrella term covering several distinct diseases, including:
| Disorder | Main Feature | Genetic Link | Notes |
|---|---|---|---|
| Mastocytosis | Too many mast cells | KIT mutation (not inherited) | Can be systemic or cutaneous |
| Mast Cell Leukemia | Rapid proliferation of atypical cells | – | Rare and aggressive |
| Mast Cell Activation Syndrome (MCAS) | Overactive mast cells | Various, under research | Symptoms across multiple organ systems |
Let’s go into the first two in a little more detail, and then focus on MCAS.
Mastocytosis: Systemic or Cutaneous
Mastocytosis is a condition caused by a proliferation of mast cells due to too many being created and released in the bone marrow. These mast cells can then accumulate in various organs. For 80%-90% of people with systemic mastocytosis, a mutation in the KIT gene is the cause. The KIT mutations are not inherited; instead, they arise spontaneously during cell division (similar to cancer mutations). The KIT gene controls mast cell creation from stem cells in the bone marrow, and mutations in KIT cause excess mast cell proliferation.[ref][ref]
A mastocytosis diagnosis is either systemic mastocytosis (affecting the whole body) or cutaneous mastocytosis, which has more skin-related symptoms. Specific markers are used to distinguish and diagnose systemic mastocytosis, including the World Health Organization criteria.
While the KIT mutations in mastocytosis are not inherited from a parent, people with mastocytosis are more likely to have a first-degree relative with it. This leads researchers to assume that there is a heritable component to mastocytosis as well (e.g., other genes involved).[ref][ref] In addition to KIT mutations, systemic mastocytosis also has links to mutations in the JAK2, PDGFRα, NRAS, RASGRP4, CBL, IL4, TET2, and IL13 genes.[ref]
Interestingly, about half of all kids with cutaneous mastocytosis tend to outgrow it and go into long-term remission. It is another reason to think that there is something else going on besides the KIT gene mutations.[ref]
Mast Cell Leukemia:
Advanced mast cell disorders include a type of leukemia called mast cell leukemia. This rare condition is caused by the creation of a large number of atypical mast cells. This type of leukemia progresses rapidly and often doesn’t have a good prognosis.[ref]
Mast Cell Activation Syndrome:
Mast Cell Activation Syndrome (MCAS) is a systemic auto-inflammatory disease that can affect multiple organs. There are a variety of clinical presentations and symptoms of this syndrome. For some people, it manifests as an almost constant allergic reaction state — like an allergy to almost everything. But for others, there are many non-allergic symptoms.
While more severe forms are not all that common, some research estimates that MCAS could “affect up to 17% of the population on a spectrum from very mild to debilitating symptoms.”[ref] Others include very mild symptoms such as allergic rhinitis in the mast cell activation spectrum, which includes up to 30% of the population.[ref]. Basically, it depends on how you define it.
In general, clinicians tend to diagnose mast cell activation syndrome in people with more severe symptoms, and patients often see multiple doctors in search of a diagnosis.
Symptoms of Mast Cell Activation Syndrome:
Symptoms of mast cell activation syndrome include:[ref]
| System | Common Symptoms |
|---|---|
| Dermatological | Swelling, flushing, itching, hives, dermatographia |
| Gastrointestinal | Abdominal pain, bloating, diarrhea, nausea, vomiting |
| Respiratory | Hoarseness, throat swelling, wheezing |
| Neurological | Headache, brain fog, neuropathy, tingling |
| Musculoskeletal | Bone/muscle pain, osteoporosis |
| Nose and Eye | Congestion, itching, watery eyes |
| Cardiovascular | Chest pain, low blood pressure, arrhythmia |
| Systemic | Anaphylaxis, fatigue |
People with MCAS will usually have some, but not all, of the above symptoms.
What are the most common symptoms of MCAS? A survey of people with a mast cell activation syndrome diagnosis found that 94% had abdominal pain, and 89% had skin-related issues (dermatographism and flushing). Other common symptoms included headache, diarrhea, and memory or concentration difficulties.[ref]
Some researchers define mast cell activation syndrome as including symptoms from at least two different organ systems. For example, someone with cardiovascular symptoms wouldn’t meet the criteria unless they also had gastrointestinal or dermatological symptoms.[ref]
One form of MCAS, now called ‘idiopathic anaphylaxis‘, involves sudden, often random-seeming episodes of throat swelling, itching, wheezing, shortness of breath, stomach pain, nausea, vomiting, diarrhea, and a drop in blood pressure. In short, an anaphylaxis episode occurs without the presence of an allergen.[ref]
Another subtype of MCAS (at least according to some researchers) is histamine intolerance. Characterized by histamine-related symptoms, consuming foods high in histamine can worsen it.
Related article: Histamine intolerance genes
Many other conditions have mast cell activation as a component, including:
- idiopathic abdominal pain
- GERD (heartburn)
- chronic urticaria
- fibromyalgia
- atypical drug and insect bite reactions
- allergies
- chronic sinusitis
- pharyngitis and vocal tension
- dermatographism
- chronic headaches
- interstitial cystitis
- atrial fibrillation
Below is an image from a recent scientific review of mast cells covering many conditions involving mast cell activation. (Creative Commons license)[ref]
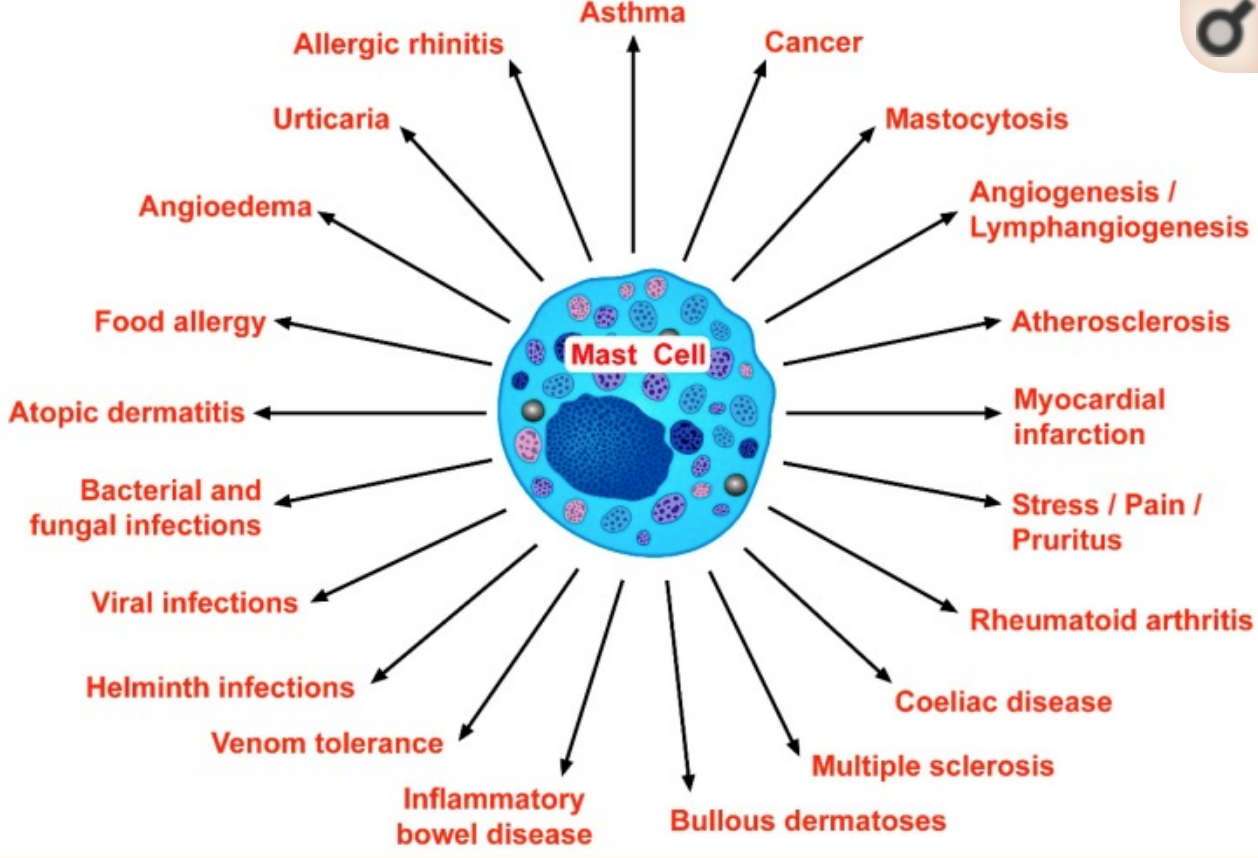
Finally, some researchers point out the growing evidence of mast cell involvement in several different autoimmune diseases, including MS, type 1 diabetes, and rheumatoid arthritis.[ref]
Mast cell triggers and mediators:
Researchers have found that there are a variety of substances within the body that will trigger mast cells to release mediators without complete degranulation. These include:[ref]
- Inflammatory cytokines (IL-1B, IL-33), which cause the release of IL-6, TNF, IL-8, and VEGF
- Heavy metals (aluminum, cadmium, mercury)
- Herbicides (atrazine, glyphosate)
- Pathogens (Lyme disease, lipopolysaccharides from bacteria, certain viruses, and mold spores)
These triggers can cause a two-phase reaction:
- Immediately released mediators include histamine, TNF, tryptase, RANKL, and serotonin. Mast cells can also synthesize and release prostaglandin D2 and leukotrienes quickly.
- Hours after the initial trigger, mast cells produce and secrete certain chemokines (TNF, CCL2, CCL8) and renin.[ref]
The different mediators released can lead to different symptoms of mast cell activation syndrome:
- Histamine leads to headaches, migraines, low blood pressure, itching, stomach acid release (and much more)
- Tryptase causes inflammation and fibrinogen lysis.
- Prostaglandin E2 also produces inflammation and pain. It can manifest itself in a variety of ways. For example, animal studies show mast cells in the colon release prostaglandin E2, causing pain and hypersensitivity in IBS (irritable bowel syndrome). Colon biopsies from IBS-D patients also showed increased levels of prostaglandin E2. Using either a mast cell stabilizer or a medication to reduce prostaglandin E2 synthesis (e.g., a COX2 inhibitor) stopped the hypersensitivity in the animal model of IBS.[ref]
In the Lifehacks section (below), you’ll find a more comprehensive list of substances that also trigger mast cell degranulation.
MRGPRX2 receptor:
Mast cells can be activated by IgE antigens in typical allergic reactions. More recently, researchers have discovered another receptor, called MRGPRX2, that reacts to protein fragments, such as in a viral infection, as well as drugs such as fluoroquinolone, opioids, and more. This receptor seems to be the key to drug hypersensitivity reactions, such as to contrast media for CT scans.
Read the full article on the MRGPRX2 receptor here.
Thyroid hormone, hypothyroidism, and mast cells:
The thyroid is a small gland in the neck that produces hormones that regulate cell metabolism, cardiac output, respiratory rate, alertness, and reproductive health. The active form of thyroid hormone, T3, binds to thyroid receptors in the cell nucleus to turn on or off genes for transcription. Thyroid hormone receptor activation affects the expression of a wide range of genes, including metabolism and energy production.[ref]
Recent research shows that mast cells have thyroid-stimulating hormone (TSH) receptors on their cell membranes. Additionally, mast cells can store thyroid hormones. Taken together, the researchers concluded that thyroid function may affect mast cells, and that mast cell degranulation could affect thyroid function.[ref] In patients with autoimmune thyroid disease, mast cells infiltrate the thyroid gland and then degranulate.[ref]
Animal studies show that hypothyroidism (low thyroid hormone levels) increases the number of mast cells by up to 4.5-fold. This also drives an increase in histamine levels by 50%. Moreover, in the hypothyroid animals, many of the mast cells showed partial or complete degranulation, which was in contrast to the non-activated mast cells in the animals with normal thyroid function.[ref] Other animal studies show that hypothyroidism increases mast cell infiltration into the liver and mast cell levels in the bone marrow. Hypothyroid animals also have prolonged wound healing due to increased mast cells.[ref][ref][ref]
Related article: Thyroid hormones, genetics, and hypothyroidism
IL-6 and Mast Cells:
IL-6 plays an interesting role here. Interleukin-6 (IL-6) is a cytokine produced by several different immune system cells in response to infection and acute inflammation. IL-6 levels become elevated in people with systemic mastocytosis, chronic urticaria, and asthma. Constant IL-6 exposure can cause the body to form more reactive mast cells. One study concludes IL-6-enhanced mast cells have “significantly enhanced FcεRI-mediated signaling, degranulation, and cytokine production”.[ref]
Asthma and mast cell activation:
Mast cells are abundant near the epithelial cells lining the lungs. Researchers theorize that asthma may be due to either inappropriate mast cell activation or that the mast cells are hypersecretory.[ref][ref]
One study sums up that mast cells release “preformed mediators including chymase, tryptase, and histamine and de novo synthesized mediators such as PGD2, LTC4, and LTE4 in addition of cytokines mainly TGFβ1, TSLP, IL-33, IL-4, and IL-13 participate in the pathogenesis of asthma.”[ref]
Related article: Asthma: understanding the genetics and underlying causes
Mast cells as a causative factor in osteoarthritis:
Osteoarthritis, the most common form of arthritis, is caused by inflammation and degeneration of the joint tissue. Researchers and doctors have known that for years – inflammation, heat, pain, and the eventual remodeling of the joint (e.g., knobby toes and gnarled fingers).
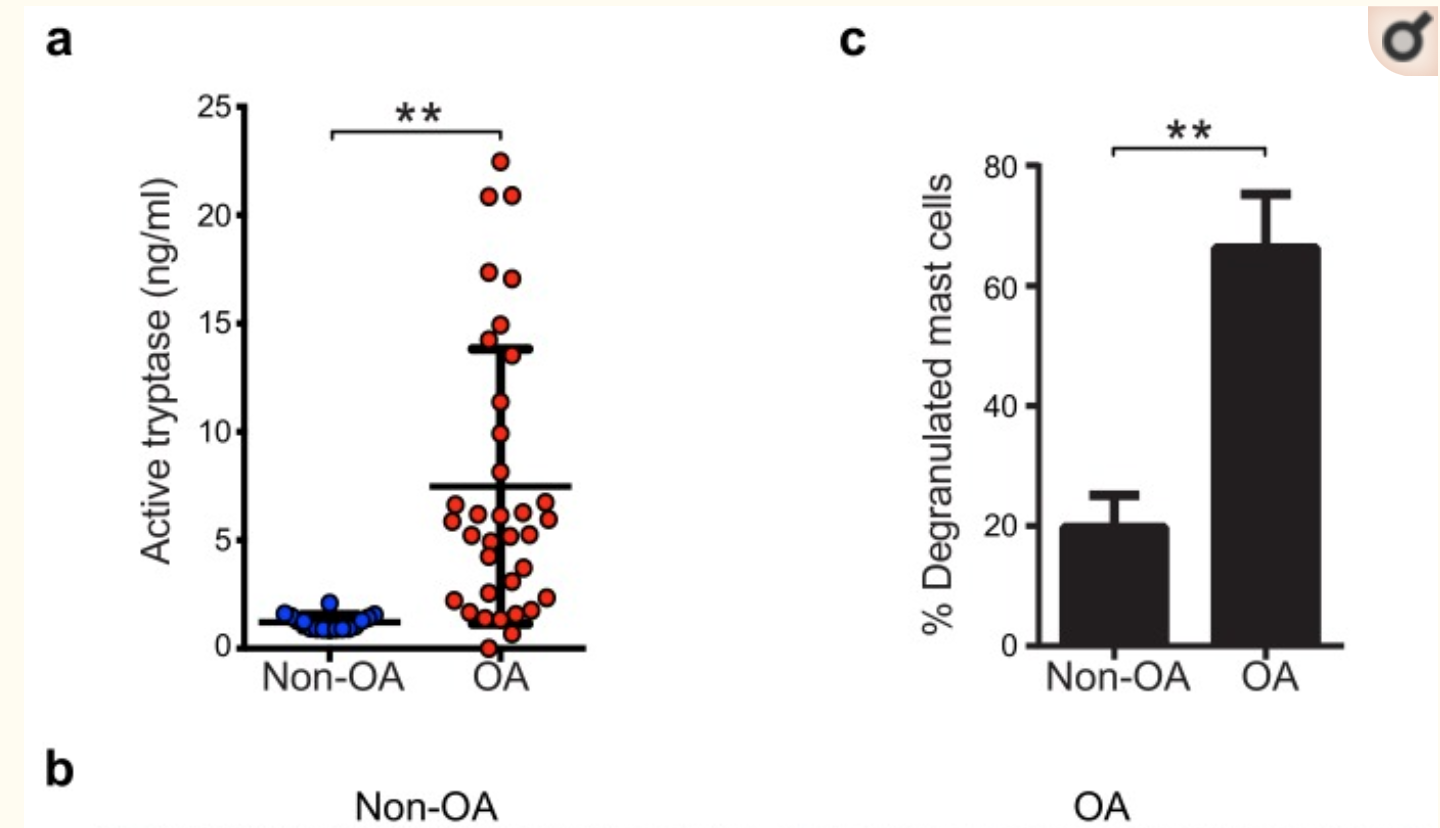
Recently, researchers have found that excessive mast cell degranulation in the joints is the cause of osteoarthritis.[ref]
To prove this, researchers looked at both human joints and genetic models using mice. They found that genetically reducing mast cells in mice completely prevented osteoarthritis. Additionally, they were able to show that activation of mast cells in osteoarthritis is through IgE/FcεRI. Going one step further, researchers showed that tryptase and the number of mast cells are both elevated in the joints of people with osteoarthritis. The activation of mast cells caused the destruction of neighboring chondrocytes and the breakdown of cartilage.[ref]
Related article: Osteoarthritis genes
The figure below is from a study on mast cells in osteoarthritis, showing both increased numbers of mast cells and an increase in tryptase. (Creative Commons License)[ref]
Chronic urticaria (hives, itchiness):
Unsurprisingly, chronic urticaria is linked to mast cell activation and histamine release. Chronic urticaria is defined as having episodes of itchy wheals or hives on the skin, with the chronic part meaning it happens (intermittently) for six months or more.
Some people with chronic urticaria have autoantibodies against the FcεRI receptor or against IgE — but it doesn’t seem to be an autoimmune condition for everyone. Regardless of the initiating cause, mast cell degranulation is involved.[ref]
The first thing most clinicians recommend for chronic urticaria is non-sedating antihistamines that block the H1 receptor. It works for a portion of patients but not for everyone. Genetics plays a role here, and people who have genetically lower IgE levels are less likely to have chronic urticaria.[ref]
Mast cells, atrial fibrillation, and cardiac diseases:
Researchers estimate that there are ~50,000 mast cells/g of human heart tissue. They lie just outside the epithelial cells that make up blood vessels and in the areas surrounding the nerves and myocytes. In heart failure and cardiomyopathy, the number of mast cells is greatly increased.[ref]
Atrial fibrillation (a-fib) is a heart arrhythmia that includes rapid atrial activation and an irregular response in the ventricle. There are several very interesting studies explaining how mast cells are involved in causing a-fib. There is fibrosis in the atrium in hearts with a-fib, with an accumulation of extracellular matrix (ECM) proteins. This increase in ECM proteins – fibrosis – separates the individual bundles of muscle cells and disturbs the impulse propagation that causes the heart to beat regularly.[ref]
Animal studies from about a decade ago show a pressure overload in the heart (stressing out the heart) can cause mast cells to proliferate. When these mast cells infiltrate the atrium, it induces PDGF-a (platelet-derived growth factor), which causes fibrosis in the mouse heart, causing a-fib. In mice bred to be deficient in mast cells (kit mutation), fibrosis and a-fib did not develop.[ref]
Related article: Atrial fibrillation genes
Mast cells are also implicated in the narrowing of the arteries due to atherosclerosis and play a role in stress-induced coronary artery disease. Oxidized LDL cholesterol can trigger mast cells.[ref][ref]
Another study on mast cells and oxidized LDL: “The results of this work indicate that the co-activation of macrophages and mast cells by oxLDL is an important mechanism for endothelial dysfunction and atherogenesis. The observed synergistic effect suggests that both macrophages and mast cells play a significant role in the early stages of atherosclerosis. Allergic patients with a lipid-rich diet may be at high risk for cardiovascular events due to high concentrations of low-density lipoprotein and histamine in arterial vessel walls.”[ref][ref]
Related article: LDL cholesterol
Interstitial cystitis as a mast cell disease:
Interstitial cystitis, or painful bladder syndrome, is a disease that feels like a constant bladder infection. One cause (among several) of interstitial cystitis is overactive mast cells in the bladder. Allergens or toxins in the urine are thought to cause the bladder walls to become sensitized to increased mast cells.[ref][ref][ref]
Vulvodynia and mast cell activation:
Vulvodynia is a chronic genital pain condition that includes increased numbers of mast cells. This condition is prevalent in about 8% of adult women, although it isn’t talked about much.[ref]
Animal studies show certain preservatives often used in body washes and shampoos can cause “increased vaginal mast cells and eosinophils and had higher serum Immunoglobulin E”. Specifically, researchers used methylisothiazolinone, found in hundreds of personal care products, including vaginal wash products. The solution here was vaginal administration of THC (the active component of marijuana) to the mice… (how would you like that job :-).[ref]
Mast cells and rheumatoid arthritis:
Rheumatoid arthritis is an autoimmune disease that causes painful inflammation of the joints. The joints of people with rheumatoid arthritis contain high levels of mast cells and other immune cells, such as T-cells and B-cells. Recent studies on rheumatoid arthritis show that it is more complex than initially thought of as an autoimmune response to certain antibodies. Mast cells are now thought to also play a pathogenic role in the condition.[ref]
Researchers have found that the average prostaglandin D2 levels (an indicator of mast cell activation) are higher in people with rheumatoid arthritis than in healthy controls.[ref]
Related article: Rheumatoid arthritis genes
Mast cells may play a role in obesity and diabetes:
It has been known for a while that obesity is linked to increased systemic inflammation. Interestingly, fat cells (adipocytes) have recently been shown to contain mast cells. One of the peptides that trigger mast cells is leptin, which is produced at higher levels in people with more fat mass. These adipocyte mast cells then release pro-inflammatory cytokines, including TNF-alpha, and increase inflammation even more. Indeed, researchers found that obese individuals have higher levels of mast cell-derived tryptase.[ref][ref]
Taking this one step further, some researchers link increased inflammation due to mast cell activation in adipose tissue as a cause of diabetes. Animal studies show that mast cell stabilizing agents (cromolyn and ketotifen) can reduce both obesity and diabetes. In mice bred to be deficient in mast cells, even an obesity-inducing diet doesn’t cause the expected weight gain.[ref][ref] Other research, though, claims mast cells have nothing to do with obesity. It depends on which genes are varied in the research animals and perhaps what makes up the high-fat diet.[ref]
Related article: Diabetes genetic susceptibility
Are mast cells at the root of everyone’s weight problem? I don’t think that is clear from the research — and there are a lot of different components of appetite regulation, fat storage, and base metabolic rate that come into play with obesity. Instead, mast cells may be what drives excess inflammation — in some people who are obese. One dietary difference in the activation of mast cells in obesity (in animal studies) is cholesterol levels. Again, this is an animal study, so it may not hold true for humans. But in animals fed a high-fat diet, mast cell inhibitors stopped the inflammation, histamine release, and weight gain. It was true, though, only in diets that contained cholesterol.[ref]
Role of mast cells in rosacea:
Rosacea is an inflammatory skin condition that plagues many people. Animal studies show mast cells are involved in inflammation, and a deficiency of mast cells caused the animals not to have rosacea. There are several mediators here that can cause mast cells to degranulate in skin tissue with rosacea. These include host defense peptides, such as LL-37, as well as substance P (pain neurotransmitter), VIP (vasoactive intestinal peptide), and serotonin. When activated, the mast cells in the skin release mediators (histamines, tryptase, TNF-alpha, CXCL9, etc.) that cause inflammation, flushing, itching, or burning.[ref]
Related article: Rosacea, underlying causes, genetic susceptibility
Dermatographism and mast cell activation syndrome:
In a survey of mast cell activation syndrome, dermatographism was found in 89% of the patients.[ref] Dermatographism is when you get welts or raised red lines when you scratch your skin lightly. It can also happen due to pressure or rubbing. It is also called dermographia, dermatographic urticaria, or skin writing, and tends to get lumped into atopic dermatitis diagnosis.[ref]
Cold urticaria (hives or welts due to cold) or cold-dependent dermatographism, another mast cell-related condition, happens when the skin is lightly scratched after being chilled.[ref]
Peripheral neuropathy and mast cell activation:
Neuropathy causes numbness, pain, pins and needles, and weakness, usually in the hands and feet. It is usually caused by damage to the peripheral nerves due to diabetes, infections, injuries, or toxins.
Mast cells are found in the area around the terminal ends of peripheral nerves. Upon degranulation, they release histamine, stimulating the nerve to release substance P and glutamate from the nerve terminals. It can actually start a cycle where the nerve releases substances that activate the mast cells, releasing mediators that activate the nerve. Researchers theorize this may be one of the causes of various chronic pain syndromes, including neuropathy.[ref][ref]
Celiac disease onset and progression:
Researchers recently found that the severity of intestinal damage in celiac disease relates to the number of mast cells in the area. Additionally, mast cells directly respond to gliadin fragments (part of the gluten protein) and release proinflammatory mediators.[ref]
Related article: Celiac Genes
Overlapping conditions with mast cell activation syndrome:
MCAS diagnosis is commonly found in several other conditions that may be interrelated or point to a single underlying cause.
Ehlers-Danlos and Mast Cell Activation:
As mentioned above, mature mast cells are found in tissues and congregate in the connective tissue around lymphatic vessels, blood vessels, nerves, and skin. One syndrome that can go hand-in-hand with mast cell activation syndrome is Ehlers-Danlos, a tissue connectivity disorder. People who have Ehler’s Danlos can have increased joint hypermobility, skin hyper-elasticity, vascular fragility, varicose veins, orthostatic intolerance, asthma, and osteoporosis. Researchers have noted an overlap that often occurs between people with Ehlers-Danlos and mast cell activation syndrome.[ref]
Related article: Check your 23andMe data for Ehlers-Danlos Syndrome genes
POTS – Postural orthostatic tachycardia syndrome:
There is an overlap in some patients between MCAS and postural orthostatic tachycardia syndrome (POTS), a common type of dysautonomia. Pretty much all the research on the topic, though, says there are a lot of unanswered questions here, and more research is needed.[ref][ref]
Related article: POTS syndrome genes
Restless Leg Syndrome:
A recent study found people with mast cell activation syndrome were about 3 to 4 times more likely than normal to have restless leg syndrome also.[ref]
Related article: Restless Leg Syndrome Genes
Let’s look at some of the genes that play a role in MCAS…
Mast Cell Genotype Report:
Lifehacks:
Below are evidence-based ways of preventing mast cells from degranulating, either by avoiding triggers or substances that block various aspects of mast cell degranulation.
If you are under a doctor’s care or taking any medications regularly, be sure to check with your doctor before making any changes.
Avoid substances that cause mast cell degranulation:
The following have been shown in studies to cause mast cells to degranulate. The majority of the studies are in cell cultures or animals, so it may not apply completely to individuals. You may want to experiment with each one to see if they cause mast cell-related symptoms for you.
- For anything you are allergic to… traditional allergy testing can help a lot here.
- “Calcium triggers the secretion of histamine from mast cells after previous exposure to sodium fluoride.”[ref] Sodium fluoride is added to drinking water in most US municipalities.
- PFOAs (Perfluorooctanoic acid) have been found to release histamine and cause mast cell degranulation. “… PFOA exacerbated allergic symptoms via hypothermia and increased serum histamine, TNF-α, IgE, and IgG1 in the ovalbumin-induced systemic anaphylaxis. The present data indicate PFOA aggravated FcɛRI-mediated mast cell degranulation and allergic symptoms.”[ref] PFOAs are found in Teflon, stain-resistant carpeting, microwave popcorn bags, and other food wrappers, etc.
- Off-gassing from carpeting may cause mast cell release in cases of sick building syndrome.[ref] (Note that this may overlap with PFOAs since they used to be used on stain-resistant carpets.
- Sodium benzoate, a common preservative, causes histamine release in people with allergies and asthma.[ref]
- Glyphosate (the active ingredient in RoundUp) has been shown to cause mast cell degranulation in the airways.[ref] In rats exposed to low-dose RoundUp, the adipose tissue and liver cells had increased inflammatory markers, including prostaglandin D2 synthesis from mast cells.[ref]
- Endosulfan, a pesticide and wood preservative, causes mast cell release.[ref]
- Methylisothiazolinone, a really common preservative used in cosmetics, shampoo, and personal care products, has been shown to increase mast cells and IgE (animal and human allergy studies).[ref][ref][ref]
- BPA exposure in utero increases mast cells in adults (animal study).[ref] Human cell studies show that low levels of BPA increase histamine and leukotriene release from mast cells.[ref] (Read more about BPA and genes)
- Mercury and silver in dental amalgam fillings have been shown to cause histamine release from mast cells.[ref]
While not a ‘substance’ per se, psychological stress causes mast cell degranulation.[ref][ref][ref]
Diet:
Allergy elimination diets and low-histamine diets have been shown in studies to be helpful to some people with chronic itching (one symptom of MCAS). Researchers recommend a 3-week trial of any dietary intervention and only trying one intervention at a time.[ref]
(Read more about histamine intolerance and a low histamine diet.)
Exercise:
In general, exercise is good for you. Lots of research shows that for the majority of people, exercise is a good thing. But… exercise causes both histamine and tryptase levels to rise. Research shows this is due to both de novo formation and mast cell degranulation. This release of histamine and tryptase increases blood flow to the skeletal muscles during exercise.[ref]
Hypoxia has also been shown to induce mast cell degranulation.[ref]
This is a normal response for most people, and the body can handle a certain increase in histamine and tryptase. Still, some people with mast cell activation syndrome may find that exercise triggers a mast cell event.
This is another one of those ‘experiment and track it’ situations: Keep track of your workouts and note any mast cell-related responses over the following 24 hours. You should dial in the right amount of exercise that causes you the least amount of mast cell degranulation.
Progesterone:
Bioidentical progesterone prevents histamine secretion from mast cells in animals.[ref] Cell line studies also show that progesterone reduces mast cell proliferation.[ref] Note that progesterone is a hormone that can affect estrogen, testosterone, and DHEA levels. Talk with your doctor before starting a supplemental hormone if you have any questions on how it will affect your overall health, reproductive health, or interact with medications.
Related article: Progesterone genes
Supplements that act as mast cell stabilizers:
Conclusion:
Mast cells touch on a lot of organ systems and pathologies in the body. The more I learn, the more amazed I am at the complex interaction between mast cells, immune responses, and systemic diseases.
Wrapping up this article, I wanted everyone to know that this is just the tip of the iceberg when it comes to information and studies about mast cells and MCAS. Topics not covered include the links between mast cells and multiple sclerosis, dementia, cancer, Crohn’s, ALS, endometriosis, Hidradenitis suppurativa, and osteoporosis.
Related Articles and Topics:
Is Inflammation Causing Your Depression and Anxiety? The Science Behind the Link
TNF-alpha: Inflammation, Chronic Diseases, and Genetic Susceptibility
References:
Agúndez, José A. G., et al. “The Diamine Oxidase Gene Is Associated with Hypersensitivity Response to Non-Steroidal Anti-Inflammatory Drugs.” PLoS ONE, vol. 7, no. 11, Nov. 2012, p. e47571. PubMed Central, https://doi.org/10.1371/journal.pone.0047571.
Andersson, Cecilia, et al. “Revisiting the Role of the Mast Cell in Asthma.” Current Opinion in Pulmonary Medicine, vol. 22, no. 1, Jan. 2016, pp. 10–17. PubMed, https://doi.org/10.1097/MCP.0000000000000228.
Arriaga-Gomez, Erica, et al. “Repeated Vaginal Exposures to the Common Cosmetic and Household Preservative Methylisothiazolinone Induce Persistent, Mast Cell-Dependent Genital Pain in ND4 Mice.” International Journal of Molecular Sciences, vol. 20, no. 21, Oct. 2019, p. 5361. PubMed Central, https://doi.org/10.3390/ijms20215361.
Baothman, Bandar K., et al. “Prostaglandin D2 Generation from Human Lung Mast Cells Is Catalysed Exclusively by Cyclooxygenase-1.” European Journal of Pharmacology, vol. 819, Jan. 2018, pp. 225–32. PubMed, https://doi.org/10.1016/j.ejphar.2017.12.005.
Bottema, Renske W. B., et al. “Interleukin 13 and Interleukin 4 Receptor-α Polymorphisms in Rhinitis and Asthma.” International Archives of Allergy and Immunology, vol. 153, no. 3, 2010, pp. 259–67. PubMed, https://doi.org/10.1159/000314366.
Cardet, Juan-Carlos, et al. “Immunology and Clinical Manifestations of Non-Clonal Mast Cell Activation Syndrome.” Current Allergy and Asthma Reports, vol. 13, no. 1, Feb. 2013, pp. 10–18. PubMed Central, https://doi.org/10.1007/s11882-012-0326-8.
Chen, Chong, and Damir B. Khismatullin. “Oxidized Low-Density Lipoprotein Contributes to Atherogenesis via Co-Activation of Macrophages and Mast Cells.” PloS One, vol. 10, no. 3, 2015, p. e0123088. PubMed, https://doi.org/10.1371/journal.pone.0123088.
Chen, J., et al. “Polymorphisms of RAD50, IL33 and IL1RL1 Are Associated with Atopic Asthma in Chinese Population.” Tissue Antigens, vol. 86, no. 6, Dec. 2015, pp. 443–47. PubMed, https://doi.org/10.1111/tan.12688.
da Silva, Elaine Zayas Marcelino, et al. “Mast Cell Function.” Journal of Histochemistry and Cytochemistry, vol. 62, no. 10, Oct. 2014, pp. 698–738. PubMed Central, https://doi.org/10.1369/0022155414545334.
Doherty, Taylor A., and Andrew A. White. “Postural Orthostatic Tachycardia Syndrome and the Potential Role of Mast Cell Activation.” Autonomic Neuroscience: Basic & Clinical, vol. 215, Dec. 2018, pp. 83–88. PubMed, https://doi.org/10.1016/j.autneu.2018.05.001.
Gao, Wei, et al. “Quercetin Ameliorates Paclitaxel-Induced Neuropathic Pain by Stabilizing Mast Cells, and Subsequently Blocking PKCε-Dependent Activation of TRPV1.” Acta Pharmacologica Sinica, vol. 37, no. 9, Sept. 2016, pp. 1166–77. PubMed, https://doi.org/10.1038/aps.2016.58.
Giannetti, Matthew P., et al. “Idiopathic Anaphylaxis: A Form of Mast Cell Activation Syndrome.” The Journal of Allergy and Clinical Immunology. In Practice, vol. 8, no. 4, Apr. 2020, pp. 1196–201. PubMed, https://doi.org/10.1016/j.jaip.2019.10.048.
Graham, Amy C., et al. “Mast Cells and Influenza A Virus: Association with Allergic Responses and Beyond.” Frontiers in Immunology, vol. 6, May 2015, p. 238. PubMed Central, https://doi.org/10.3389/fimmu.2015.00238.
Gupta, Kalpna, and Ilkka T. Harvima. “Mast Cell-Neural Interactions Contribute to Pain and Itch.” Immunological Reviews, vol. 282, no. 1, Mar. 2018, pp. 168–87. PubMed Central, https://doi.org/10.1111/imr.12622.
—. “Mast Cell-Neural Interactions Contribute to Pain and Itch.” Immunological Reviews, vol. 282, no. 1, Mar. 2018, pp. 168–87. PubMed Central, https://doi.org/10.1111/imr.12622.
Haenisch, Britta, et al. “Systemic Mast Cell Activation Disease: The Role of Molecular Genetic Alterations in Pathogenesis, Heritability and Diagnostics.” Immunology, vol. 137, no. 3, Nov. 2012, pp. 197–205. PubMed Central, https://doi.org/10.1111/j.1365-2567.2012.03627.x.
Hon, Yuen Yi, et al. “Endogenous Histamine and Cortisol Levels in Subjects with Different Histamine N-Methyltransferase C314T Genotypes.” Molecular Diagnosis & Therapy, vol. 10, no. 2, 2006, pp. 109–14. PubMed Central, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4178529/.
Jain, Preetesh, et al. “Mast Cell Leukemia (MCL): Clinico-Pathologic and Molecular Features and Survival Outcome.” Leukemia Research, vol. 59, Aug. 2017, pp. 105–09. PubMed Central, https://doi.org/10.1016/j.leukres.2017.05.018.
Kim, Do-Kyun, et al. “Aldh2 Attenuates Stem Cell Factor/Kit-Dependent Signaling and Activation in Mast Cells.” International Journal of Molecular Sciences, vol. 20, no. 24, Dec. 2019, p. 6216. PubMed Central, https://doi.org/10.3390/ijms20246216.
—. “Aldh2 Attenuates Stem Cell Factor/Kit-Dependent Signaling and Activation in Mast Cells.” International Journal of Molecular Sciences, vol. 20, no. 24, Dec. 2019, p. 6216. PubMed Central, https://doi.org/10.3390/ijms20246216.
Kim, Hui-Hun, et al. “Vigna Angularis Inhibits Mast Cell-Mediated Allergic Inflammation.” International Journal of Molecular Medicine, vol. 32, no. 3, Sept. 2013, pp. 736–42. PubMed, https://doi.org/10.3892/ijmm.2013.1430.
Kim, Tae-Ho, et al. “The Inhibitory Effect of Naringenin on Atopic Dermatitis Induced by DNFB in NC/Nga Mice.” Life Sciences, vol. 93, no. 15, Oct. 2013, pp. 516–24. PubMed, https://doi.org/10.1016/j.lfs.2013.07.027.
Krystel-Whittemore, Melissa, et al. “Mast Cell: A Multi-Functional Master Cell.” Frontiers in Immunology, vol. 6, Jan. 2016, p. 620. PubMed Central, https://doi.org/10.3389/fimmu.2015.00620.
Liao, Chien-hui, et al. “Cardiac Mast Cells Cause Atrial Fibrillation through PDGF-A–Mediated Fibrosis in Pressure-Overloaded Mouse Hearts.” The Journal of Clinical Investigation, vol. 120, no. 1, Jan. 2010, pp. 242–53. PubMed Central, https://doi.org/10.1172/JCI39942.
Lyons, Jonathan J. “Hereditary Alpha Tryptasemia: Genotyping and Associated Clinical Features.” Immunology and Allergy Clinics of North America, vol. 38, no. 3, Aug. 2018, pp. 483–95. PubMed Central, https://doi.org/10.1016/j.iac.2018.04.003.
Maintz, L., et al. “Association of Single Nucleotide Polymorphisms in the Diamine Oxidase Gene with Diamine Oxidase Serum Activities.” Allergy, vol. 66, no. 7, July 2011, pp. 893–902. PubMed, https://doi.org/10.1111/j.1398-9995.2011.02548.x.
Matloubi, Mojdeh, et al. “The Impact of Interleukin (IL)-33 Gene Polymorphisms and Environmental Factors on Risk of Asthma in the Iranian Population.” Lung, vol. 198, no. 1, Feb. 2020, pp. 105–12. PubMed, https://doi.org/10.1007/s00408-019-00301-9.
—. “The Impact of Interleukin (IL)-33 Gene Polymorphisms and Environmental Factors on Risk of Asthma in the Iranian Population.” Lung, vol. 198, no. 1, Feb. 2020, pp. 105–12. PubMed, https://doi.org/10.1007/s00408-019-00301-9.
Min, Hong Ki, et al. “Roles of Mast Cells in Rheumatoid Arthritis.” The Korean Journal of Internal Medicine, vol. 35, no. 1, Jan. 2020, pp. 12–24. PubMed Central, https://doi.org/10.3904/kjim.2019.271.
Niwa, Y., et al. “FcepsilonRIalpha Gene (FCER1A) Promoter Polymorphisms and Total Serum IgE Levels in Japanese Atopic Dermatitis Patients.” International Journal of Immunogenetics, vol. 37, no. 2, Apr. 2010, pp. 139–41. PubMed, https://doi.org/10.1111/j.1744-313X.2010.00901.x.
Palikhe, Sailesh, et al. “Association Between PTPN22 Polymorphisms and IgE Responses to Staphylococcal Superantigens in Chronic Urticaria.” Allergy, Asthma & Immunology Research, vol. 7, no. 3, May 2015, pp. 290–94. PubMed Central, https://doi.org/10.4168/aair.2015.7.3.290.
—. “Association Between PTPN22 Polymorphisms and IgE Responses to Staphylococcal Superantigens in Chronic Urticaria.” Allergy, Asthma & Immunology Research, vol. 7, no. 3, May 2015, pp. 290–94. PubMed Central, https://doi.org/10.4168/aair.2015.7.3.290.
Ravanbakhsh, Naseem, and Anil Kesavan. “The Role of Mast Cells in Pediatric Gastrointestinal Disease.” Annals of Gastroenterology, vol. 32, no. 4, 2019, pp. 338–45. PubMed Central, https://doi.org/10.20524/aog.2019.0378.
Schaubschläger, W. W., et al. “Release of Mediators from Human Gastric Mucosa and Blood in Adverse Reactions to Benzoate.” International Archives of Allergy and Applied Immunology, vol. 96, no. 2, 1991, pp. 97–101. PubMed, https://doi.org/10.1159/000235478.
Skaper, Stephen D., et al. “An Inflammation-Centric View of Neurological Disease: Beyond the Neuron.” Frontiers in Cellular Neuroscience, vol. 12, Mar. 2018, p. 72. PubMed Central, https://doi.org/10.3389/fncel.2018.00072.
Talei, Mahsa, et al. “Interleukin-33 Gene Expression and Rs1342326 Polymorphism in Behçet’s Disease.” Immunology Letters, vol. 212, Aug. 2019, pp. 120–24. PubMed, https://doi.org/10.1016/j.imlet.2018.11.005.
Theoharides, Theoharis C., et al. “Recent Advances in Our Understanding of Mast Cell Activation – or Should It Be Mast Cell Mediator Disorders?” Expert Review of Clinical Immunology, vol. 15, no. 6, June 2019, pp. 639–56. PubMed Central, https://doi.org/10.1080/1744666X.2019.1596800.
—. “Recent Advances in Our Understanding of Mast Cell Activation – or Should It Be Mast Cell Mediator Disorders?” Expert Review of Clinical Immunology, vol. 15, no. 6, June 2019, pp. 639–56. PubMed Central, https://doi.org/10.1080/1744666X.2019.1596800.
Valdes, Ana M., et al. “Genome-Wide Association Scan Identifies a Prostaglandin-Endoperoxide Synthase 2 Variant Involved in Risk of Knee Osteoarthritis.” American Journal of Human Genetics, vol. 82, no. 6, June 2008, pp. 1231–40. PubMed, https://doi.org/10.1016/j.ajhg.2008.04.006.
Valent, Peter. “KIT D816V and the Cytokine Storm in Mastocytosis: Production and Role of Interleukin-6.” Haematologica, vol. 105, no. 1, Jan. 2020, pp. 5–6. PubMed Central, https://doi.org/10.3324/haematol.2019.234864.
Vanuytsel, Tim, et al. “Psychological Stress and Corticotropin-Releasing Hormone Increase Intestinal Permeability in Humans by a Mast Cell-Dependent Mechanism.” Gut, vol. 63, no. 8, Aug. 2014, pp. 1293–99. PubMed, https://doi.org/10.1136/gutjnl-2013-305690.
VCV000013852.6 – ClinVar – NCBI. https://www.ncbi.nlm.nih.gov/clinvar/variation/13852/. Accessed 19 Nov. 2021.
Walsh, Sarah K., et al. “Acute Administration of Cannabidiol in Vivo Suppresses Ischaemia-Induced Cardiac Arrhythmias and Reduces Infarct Size When given at Reperfusion.” British Journal of Pharmacology, vol. 160, no. 5, July 2010, pp. 1234–42. PubMed, https://doi.org/10.1111/j.1476-5381.2010.00755.x.
Wang, Junru, et al. “Palmitoylethanolamide Regulates Development of Intestinal Radiation Injury in a Mast Cell-Dependent Manner.” Digestive Diseases and Sciences, vol. 59, no. 11, Nov. 2014, pp. 2693–703. PubMed, https://doi.org/10.1007/s10620-014-3212-5.
Wang, Qian, et al. “IgE-Mediated Mast Cell Activation Promotes Inflammation and Cartilage Destruction in Osteoarthritis.” ELife, vol. 8, May 2019, p. e39905. PubMed, https://doi.org/10.7554/eLife.39905.
Weidinger, Stephan, et al. “Genome-Wide Scan on Total Serum IgE Levels Identifies FCER1A as Novel Susceptibility Locus.” PLoS Genetics, vol. 4, no. 8, Aug. 2008, p. e1000166. PubMed Central, https://doi.org/10.1371/journal.pgen.1000166.
Weinstock, Leonard B., et al. “Restless Legs Syndrome Is Associated with Mast Cell Activation Syndrome.” Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine, vol. 16, no. 3, Mar. 2020, pp. 401–08. PubMed, https://doi.org/10.5664/jcsm.8216.
Żelechowska, P., et al. “Mast Cells Participate in Chronic Low-Grade Inflammation within Adipose Tissue.” Obesity Reviews: An Official Journal of the International Association for the Study of Obesity, vol. 19, no. 5, May 2018, pp. 686–97. PubMed, https://doi.org/10.1111/obr.12670.
Zhang, Xian, et al. “Dietary Cholesterol Is Essential to Mast Cell Activation and Associated Obesity and Diabetes in Mice.” Biochimica Et Biophysica Acta. Molecular Basis of Disease, vol. 1865, no. 6, June 2019, pp. 1690–700. PubMed, https://doi.org/10.1016/j.bbadis.2019.04.006.
Zhou, Dongchen, et al. “Association between Chymase Gene Polymorphisms and Atrial Fibrillation in Chinese Han Population.” BMC Cardiovascular Disorders, vol. 19, no. 1, Dec. 2019, p. 321. PubMed, https://doi.org/10.1186/s12872-019-01300-7.