Key Takeaways:
~ Inclusion body myositis is a progressive chronic condition that causes muscle weakness from inflammation in the muscles.
~ Inclusion body myositis can be caused by misfolded proteins, autoimmune diseases, and genetic variants.
<b>Members</b> will see their genotype report below and the solutions in the Lifehacks section. <a href=”https://www.geneticlifehacks.com/membership/”>Consider joining today</a>.
What is inclusion body myositis?
Inclusion body myositis is a progressive, chronic condition that causes muscle weakness. The muscle weakness slowly progresses and causes difficulty climbing stairs, walking, lifting things, and swallowing.
Men are three times more likely to have inclusion body myositis than women, and symptoms often begin in the 50s or early 60s. While a rare condition, it is more commonly found in people in Norway, Western Australia, Minnesota (US), and Japan.[ref]
Sporadic inclusion body myositis:
Inclusion body myositis (IBM) is an inflammatory myopathy, which means it is a disorder that causes inflammation in the muscles. (“Myo” = muscles; “itis” = inflammation)[ref]
The term ‘sporadic’ here means it arises randomly or due to a currently unknown cause. It differentiates the later in life form of inclusion body myositis from the inherited form that tends to occur earlier in life. (More on the inherited form below.)
The main initial symptoms of inclusion body myositis are weakness in the fingers and wrists and weakness in the quadriceps and ankles. Initial diagnosis can be difficult and often misdiagnosed as arthritis or polymyositis.[ref] Additionally, muscles involving swallowing and facial movement can be impacted.[ref]
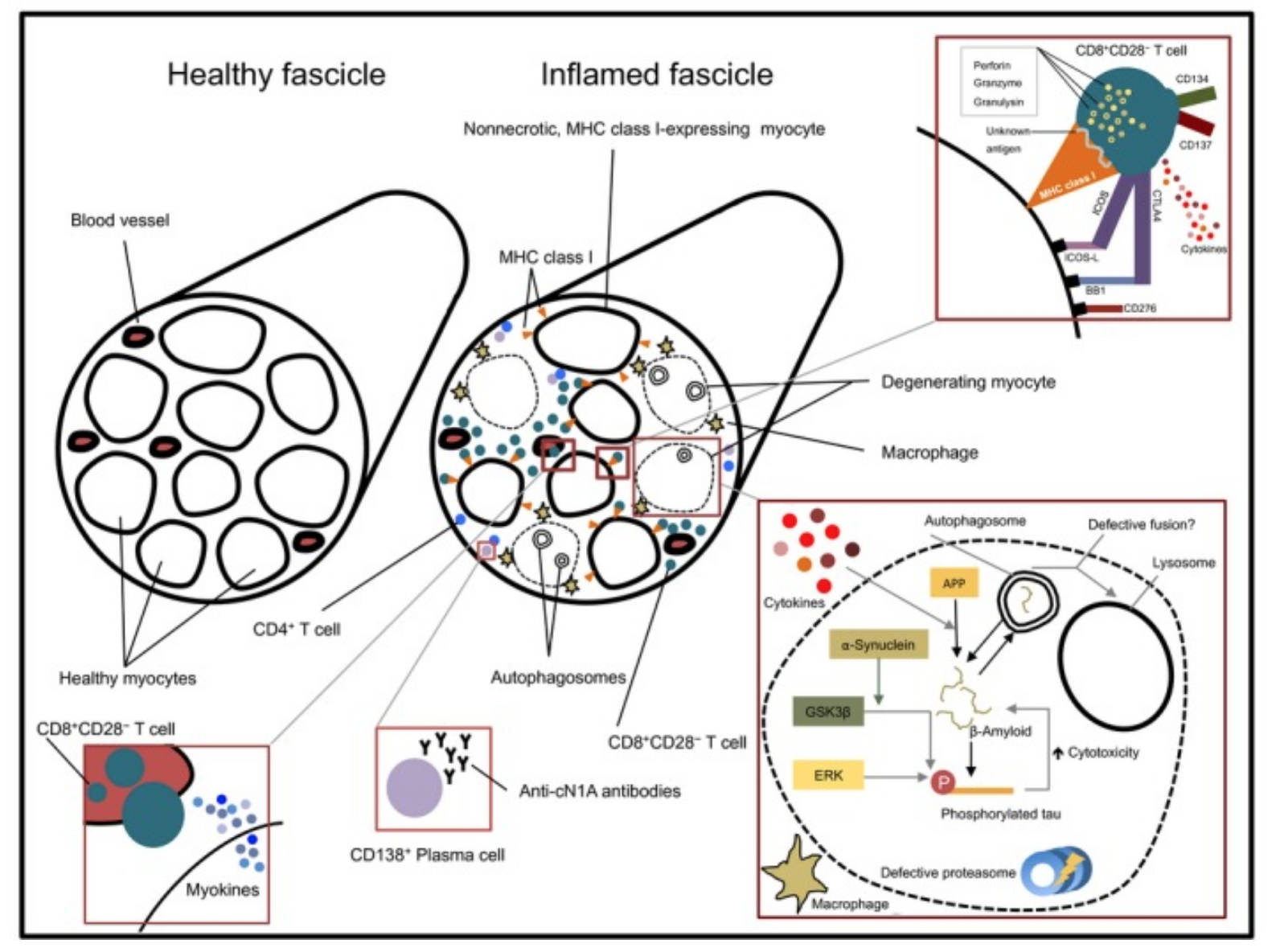
Muscle tissue changes in inclusion body myositis:
Biopsies of the muscles impacted by IBM showed inflammation surrounding the muscle fibers. Macrophages and T cells invade the muscle fibers. Additionally, there can be mitochondrial changes in the muscle fibers as well as atrophic fibers.[ref]
Another hallmark of IBM is the formation of rimmed vacuoles and the accumulation of misfolded proteins. It points to an increase in autophagy pathways.[ref]
This image from a good overview of IBM shows the changes in the muscle tissue (creative commons license):
Misfolded proteins:
Similar to age-related neurodegenerative diseases, the muscle tissue in people with IBM shows an accumulation of amyloid-beta protein, tau tangles, and alpha-synuclein. These proteins are ‘myotoxic‘ in the muscle tissue. When accumulated in the brain, these proteins cause Alzheimer’s and Parkinson’s diseases.[ref]
Increased autophagy:
Autophagy is how the body breaks down and recycles different components in the cell. It is often likened to taking out the trash and recycling. The overexpression of proteins associated with autophagy is found in muscle biopsies of sporadic inclusion body myositis.[ref]
In addition to recycling and removing misfolded proteins, defective mitochondria also break down via autophagy. Research is still ongoing on how and why autophagy is disordered in IBM.
Autoimmune or degenerative disease?
It is not yet clear to researchers whether IBM (inclusion body myositis) is an autoimmune disease – or – if it is a degenerative disease with inflammation.[ref]
Some researchers believe it is an autoimmune disease because people with IBM often have T cell abnormalities. Genetic research shows that the sporadic form of IBM is associated with known risk factors for autoimmune diseases.[ref]
Bringing this all together:
Like many complex diseases, there is likely not a simple answer to the cause of IBM. Instead, it may be an interplay between inflammation, the dysregulation of proteins, mitochondrial dysfunction, and changes to autophagy. Somewhere in the mix, autoimmunity may be triggering it.
Hereditary form: Inclusion body myopathy 2
Inclusion body myopathy 2 is a genetic form of the disease caused by mutations in the GNE gene. The GNE gene codes for an enzyme essential for creating sialic acid. People with two copies of mutations in the GNE gene can have lower levels of a type of sialic acid needed to produce certain cell-signaling proteins in muscle cells.[ref][ref]
Compared to the sporadic form, the disease’s familial (genetic) form can start affecting an individual in early adulthood.
Inflammation is not a big component of the hereditary form of inclusion body myopathy 2. Instead, the muscle biopsies show small fibers as well as protein misfolding.[ref]
Inclusion Body Myositis Genotype Report
Members: Log in to see your data below.
Not a member? Join here.
Why is this section is now only for members? Here’s why…
Lifehacks:
If you have inclusion body myositis, talk to your doctor before making any changes to your diet or lifestyle.
Lifestyle Changes:
Exercise:
Several small studies on mild to moderate exercise show that it may help prevent some of the loss of muscle strength in people with IBM.[ref]
Ketogenic diet:
A case study of a 54-year-old woman with IBM showed that a low-carb, high-fat ketogenic diet helped to maintain strength, improve walking, and increase the quality of life.[ref]
Overall healthy diet:
The Myositis Association recommends a ‘healthy diet’ as necessary… Their recommendations include avoiding processed food, reducing sugar and flour intake, and eating vegetables and fish.[ref] (To me, this seems to be an obvious starting point that most people with IBM are likely already doing.)
Supplements for inclusion body myositis:
Related Article and Topics:
Autophagy genes
Autophagy is a general term for cellular pathways that move something from the cytoplasm of the cell into the lysosome for degradation.
TNF-alpha and Inflammation
Tumor necrosis factor (TNF) is an inflammatory cytokine that acts as a signaling molecule in our immune system. In an acute inflammatory situation, TNF-alpha plays an essential role in protecting us, but genetically higher TNF-alpha levels are also linked to chronic inflammatory diseases.
AGEs and RAGEs
The receptor for Advanced Glycation Endproducts (RAGEs) is a target for HMGB1
Rapamycin, mTOR, and Your Genes
Learn about the recent research on rapamycin and how your genetic variants impact mTOR.
References:
Alfano, Lindsay N., and Linda P. Lowes. “Emerging Therapeutic Options for Sporadic Inclusion Body Myositis.” Therapeutics and Clinical Risk Management, vol. 11, Sept. 2015, pp. 1459–67. PubMed Central, https://doi.org/10.2147/TCRM.S65368.
Bayraktar, Oznur, et al. “IBMPFD Disease-Causing Mutant VCP/P97 Proteins Are Targets of Autophagic-Lysosomal Degradation.” PLoS ONE, vol. 11, no. 10, Oct. 2016, p. e0164864. PubMed Central, https://doi.org/10.1371/journal.pone.0164864.
Britson, Kyla A., et al. “New Developments in the Genetics of Inclusion Body Myositis.” Current Rheumatology Reports, vol. 20, no. 5, Apr. 2018, p. 26. PubMed Central, https://doi.org/10.1007/s11926-018-0738-0.
“Diet and Nutrition.” The Myositis Association, https://www.myositis.org/about-myositis/treatment-disease-management/complementary-and-self-care-therapies/diet-and-nutrition/. Accessed 27 June 2022.
Fernández-Martínez, J., et al. “Robust Sampling of Altered Pathways for Drug Repositioning Reveals Promising Novel Therapeutics for Inclusion Body Myositis.” Journal of Rare Diseases Research & Treatment, 2019. Semantic Scholar, https://doi.org/10.29245/2572-9411/2019/2.1174.
Girolamo, F., et al. “Overexpression of Autophagic Proteins in the Skeletal Muscle of Sporadic Inclusion Body Myositis.” Neuropathology and Applied Neurobiology, vol. 39, no. 7, Dec. 2013, pp. 736–49. PubMed, https://doi.org/10.1111/nan.12040.
—. “Overexpression of Autophagic Proteins in the Skeletal Muscle of Sporadic Inclusion Body Myositis.” Neuropathology and Applied Neurobiology, vol. 39, no. 7, Dec. 2013, pp. 736–49. PubMed, https://doi.org/10.1111/nan.12040.
Greenberg, Steven A. “Inclusion Body Myositis: Clinical Features and Pathogenesis.” Nature Reviews Rheumatology, vol. 15, no. 5, May 2019, pp. 257–72. www.nature.com, https://doi.org/10.1038/s41584-019-0186-x.
Güttsches, Anne-Katrin, et al. “Proteomics of Rimmed Vacuoles Define New Risk Allele in Inclusion Body Myositis.” Annals of Neurology, vol. 81, no. 2, Feb. 2017, pp. 227–39. PubMed Central, https://doi.org/10.1002/ana.24847.
Huizing, Marjan, and Donna M. Krasnewich. “Hereditary Inclusion Body Myopathy: A Decade of Progress.” Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease, vol. 1792, no. 9, Sept. 2009, pp. 881–87. ScienceDirect, https://doi.org/10.1016/j.bbadis.2009.07.001.
“Inclusion-Body Myositis (IBM) – Diseases.” Muscular Dystrophy Association, 18 Dec. 2015, https://www.mda.org/disease/inclusion-body-myositis.
Keller, Christian W., et al. “Immune and Myodegenerative Pathomechanisms in Inclusion Body Myositis.” Annals of Clinical and Translational Neurology, vol. 4, no. 6, May 2017, pp. 422–45. PubMed Central, https://doi.org/10.1002/acn3.419.
Mendell, Jerry R., et al. “Follistatin Gene Therapy for Sporadic Inclusion Body Myositis Improves Functional Outcomes.” Molecular Therapy, vol. 25, no. 4, Apr. 2017, pp. 870–79. ScienceDirect, https://doi.org/10.1016/j.ymthe.2017.02.015.
Muth, Ingrid E., et al. “HMGB1 and RAGE in Skeletal Muscle Inflammation: Implications for Protein Accumulation in Inclusion Body Myositis.” Experimental Neurology, vol. 271, Sept. 2015, pp. 189–97. ScienceDirect, https://doi.org/10.1016/j.expneurol.2015.05.023.
Naddaf, Elie, et al. “Inclusion Body Myositis: Update on Pathogenesis and Treatment.” Neurotherapeutics, vol. 15, no. 4, Oct. 2018, pp. 995–1005. Springer Link, https://doi.org/10.1007/s13311-018-0658-8.
Needham, Merrilee, and Frank L. Mastaglia. “Inclusion Body Myositis.” International Neurology, edited by Robert P. Lisak et al., John Wiley & Sons, Ltd, 2016, pp. 505–07. DOI.org (Crossref), https://doi.org/10.1002/9781118777329.ch122.
Nemunaitis, Gregory, et al. “Hereditary Inclusion Body Myopathy: Single Patient Response to Intravenous Dosing of GNE Gene Lipoplex.” Human Gene Therapy, vol. 22, no. 11, Nov. 2011, pp. 1331–41. liebertpub.com (Atypon), https://doi.org/10.1089/hum.2010.192.
NM_001128227.2(GNE):C.1985C>T (p.Ala662Val) AND Sialuria – ClinVar – NCBI. https://www.ncbi.nlm.nih.gov/clinvar/RCV000265100.1/. Accessed 27 June 2022.
Phillips, Matthew C. L., et al. “Impact of a Ketogenic Diet on Sporadic Inclusion Body Myositis: A Case Study.” Frontiers in Neurology, vol. 11, 2020. Frontiers, https://www.frontiersin.org/article/10.3389/fneur.2020.582402.
Rojana-udomsart, Arada, et al. “Analysis of HLA-DRB3 Alleles and Supertypical Genotypes in the MHC Class II Region in Sporadic Inclusion Body Myositis.” Journal of Neuroimmunology, vol. 254, no. 1–2, Jan. 2013, pp. 174–77. PubMed, https://doi.org/10.1016/j.jneuroim.2012.09.003.
VCV000008474.21 – ClinVar – NCBI. https://www.ncbi.nlm.nih.gov/clinvar/variation/8474/. Accessed 27 June 2022.