Key takeaways:
~ Autophagy is a cellular process where autophagosomes form around damaged components like proteins, lipids, and even malfunctioning mitochondria. These damaged components are then recycled by the cell.
~ Autophagy clears out the cellular junk, recycling it and cleaning up the cell.
~ Efficient autophagy is essential for overall cellular health. It helps mitigate oxidative stress by disposing of faulty mitochondria and reduces the risk of neurodegenerative diseases by clearing harmful proteins.
~ Genetic variants related to mTOR and autophagy-related proteins can affect your risk for chronic health conditions.
What is autophagy and why is it so important?
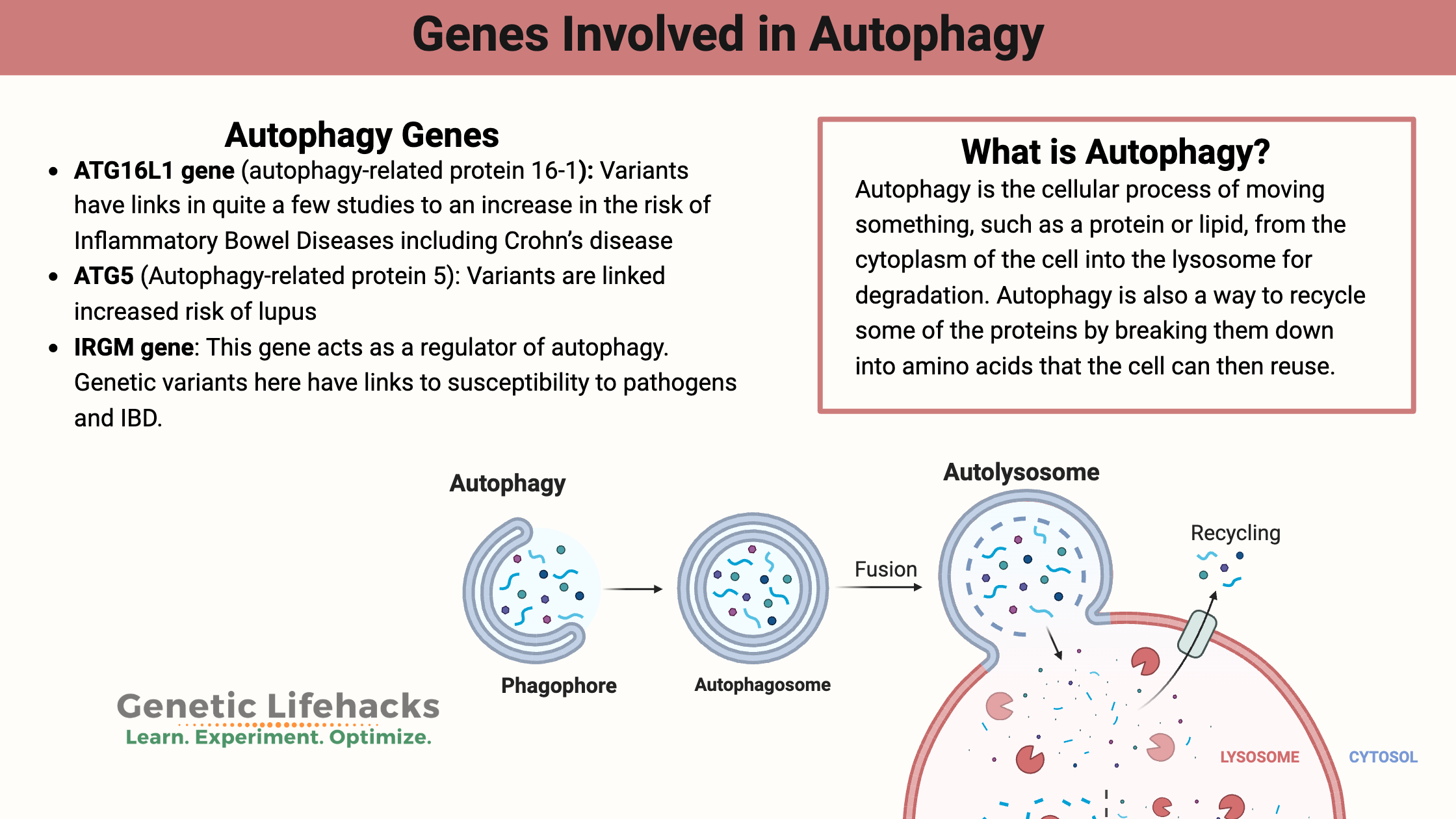
Autophagy is the cellular process of moving something, such as a protein or lipid, from the cytoplasm of the cell into the lysosome for degradation. The term comes from the Greek ‘auto’ (self) and ‘-phagy’ (to eat).
Back to high school science class: the lysosome organelle is inside almost every cell in the body. It is made up of a membrane that surrounds a bunch of different enzymes for breaking down proteins. This is a way our cells can clean up after themselves and also how they get rid of foreign invaders like bacteria.
The autophagy pathway involves forming an organelle called an autophagosome, which is a vesicle that engulfs the things inside a cell that need to be gotten rid of. My mental image is that the autophagosome is like a trashbag forming around the cellular junk that needs to be taken out. Then that trashbag (autophagosome) meets up with the lysosome, which is like throwing the trash into a big vat of bubbling acid (yep, I watched too much TV as a kid) with enzymes in it that break apart the trash. Perhaps add in a bit of chomping PacMan action to complete the mental picture?
So what kind of “junk” is in our cells? This can be anything from unused or misfolded proteins to lipids to damaged organelles such as damaged mitochondria. Autophagy – or the creation of an autophagosome around the damaged organelle – then leads to its disposal via the lysosome. Additionally, intracellular bacteria and viruses are also engulfed and then destroyed (a specific type of autophagy called xenophagy).
Autophagy is also a way to recycle some of the proteins by breaking them down into amino acids that the cell can then reuse. Thus, autophagy is normally triggered when our cells are stressed, such as when we are in starvation mode, or there is cellular stress from exposure to a toxin.[ref]
How does autophagy work?
Let me make sure this is clear with all the info about degradation and destruction: autophagy is considered to be a cytoprotective process – protective of the cell. It’s a good thing! Back to the analogy – taking out the trash and recycling the plastic bottles makes your home a better place. This is a natural part of our body’s cellular processes.[ref]
Zooming back out to the big picture here: why do we care about autophagy? It is important in a couple of ways:
- First, when the mitochondria in a cell stop functioning optimally, they need to be destroyed so that new mitochondria can be formed. Also, mitochondria that aren’t functioning optimally are more likely to create reactive oxygen species (ROS) which can cause cellular damage.
- Second, a decrease in or problem with autophagy is associated with neurogenerative diseases such as Parkinson’s and Alzheimer’s.[ref] The proteins that cause these neurodegenerative diseases can be cleared in part through autophagy.
- Additionally, autophagy plays a role in clearing intracellular (inside the cell) pathogens. This comes into play with inflammatory bowel diseases where genetic problems with autophagy have links to increased risk of IBD.
- Finally, autophagy also plays a role in hair loss[ref] and the aging effects on the skin.[ref] Age spots and lighter patches are likely due to autophagy not happening efficiently enough in skin cells.
Like most cellular processes, there is an optimal window of activity. Too little autophagy leaves a ‘dirty house’ and too much can lead to cell death.
Regulators of autophagy:
Several factors come into play here to regulate autophagy.
mTOR (mammalian or mechanistic target of rapamycin) is one of the negative regulators of autophagy: a decrease in mTOR causes an increase in autophagy.
Insulin-like Growth Factor 1 (IGF1) causes a decrease in mTOR activity under conditions of mild starvation. This then upregulates the creation of autophagosomes. A
dditionally, autophagy can be activated by AMPK, which increases with which can directly activate autophagy and also inhibits mTOR.
What is mTOR?
Basically, it is the signaling pathway that tells cells that it is time for growth. mTOR is activated when there is plenty of A/TP and amino acids (especially leucine and glutamine) available in the cell.[ref] This then signals cell growth.
There are tradeoffs, though, when it comes to the constant activation of mTOR. These questions come into play when looking at its role in cancer (when you don’t want cell growth), as well as the necessary function of autophagy for getting rid of defective mitochondria, etc.
Our body needs a balance between anabolic processes (signaled by mTOR) and catabolic processes (such as autophagy).
What induces autophagy?
Fasting – and a lack of specific amino acids – induces autophagy. This would have been a natural state of the body in the past when food wasn’t readily available at all times. Back when there wasn’t a 24-hour McDonald’s drive-thru available, people sometimes went hungry for a little bit! So it seems that our bodies are naturally prone to balancing out the times that we build up cells with the times that we clean up and break down unneeded components.
Genetics and Autophagy:
More than 30 different genes code for the proteins that form the autophagosome. Researchers are still actively figuring out how all of the bits and pieces of the process go together, but recent genetic studies have shed a lot of light on the pathway.
Genetic mutations in autophagy genes are linked with several chronic diseases and neurodegenerative disorders, showing that autophagy is an important process in preventing these diseases.[ref]
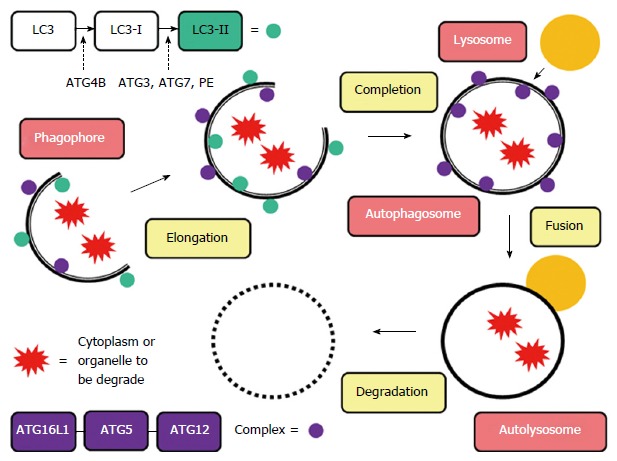
A family of genes known as the autophagy-related genes, whose abbreviations start with ATG, codes for several of the proteins integral to autophagy. Several of these genes have variants that have been studied in reference to pathogen susceptibility, autoimmune diseases, cancer, and sepsis.
ATG16L1 gene (autophagy-related protein 16-1):
ATG16L1 is necessary for the initiation of the process to create an autophagosome as well as being integral to the process of closing the membrane.[ref]
ATG16L1 genetic variants have links in quite a few studies to an increase in the risk of Inflammatory Bowel Diseases including Crohn’s disease. One theory on why the autophagy variant is a risk in Crohn’s is that it causes a decreased clearance of bacteria in the cells lining the intestine.[ref]
ATG5 (Autophagy-related protein 5):
ATG5 is a key protein in the autophagy process, specifically in the formation of the autophagosome. It interacts with other autophagy-related proteins, such as ATG12 and ATG16L1, to form the ATG12-ATG5-ATG16L1 complex.
IRGM (Immunity-Related GTPase M) is a protein that plays a key role in regulating autophagy and immune responses, particularly in the context of infection and cellular stress. IRGM is specifically involved in clearing out intracellular pathogens through xenophagy. IRGM interacts with AGT5.
Autophagy Genotype Report:
Lifehacks for increasing autophagy
If you carry some of the autophagy-related genetic variants above — or you just want to make sure that you clear out old mitochondria for optimal functioning — here are some research-backed ways of increasing autophagy.
Fasting:
A fasting-mimicking diet is a low-calorie, low-protein diet that is thought to increase autophagy and protect against toxins, improves health, and protect against diabetes, cancer, and heart disease.[ref] Read more about the fasting-mimicking diet in the book The Longevity Diet.
Intermittent Fasting
Intermittent fasting decreases mTOR and increases autophagy.[ref][ref]
There are various ways that people define intermittent fasting. For some people, it is fasting for a whole day once or twice a week. For others, it is taking an 18 to 24-hour break from eating.
Exercise:
Research shows that exercise can increase autophagy.[ref] A recent study found that the autophagy benefits are seen with more intensive exercise that is done in a fasted state.[ref] One more good reason to get active first thing in the morning!
Cold Exposure:
A study from November 2024 looked at how 7 days of “7-day cold-water acclimation” affects autophagy in young males. The researches analyzed proteins associated with autophagy, apoptosis, heat shock response, and inflammation. The study found that cold acclimation enhanced autophagic responses. [ref]
Supplements and dietary changes for increasing autophagy:
*Always check to make sure supplements don’t interact with any medications you are taking.
Related Articles and Genes:
Top 10 Genes to Check in Your Genetic Raw Data
Wondering what is actually important in your genetic data? These 10 genes have important variants with a big impact on health. Check your genes (free article).
NAD+: Research on nicotinamide riboside and NMN
Explore the research about how nicotinamide riboside (NR) and NMN are being used to reverse aging. Learn about how your genes naturally affect your NAD+ levels and how this interacts with the aging process.
Fisetin: Anti-aging and antioxidant
Recent research on the flavanoid fisetin shows that it may be a potent supplement for improving health in aging. Fisetin acts as a senolytic, clearing out senescent cells in animal and cell studies.