Key takeaways:
~ CCR5 is a receptor on immune cells.
~ A mutation in CCR5 called the Delta32 mutation blocks the AIDS virus from infecting cells.
This article explains the CCR5 gene and the Delta32 variant that protects some people from AIDS. I’ll dive into the science of how HIV infects cells and then explain how to check your 23andMe or other genetic raw data to see if you carry the variant.
Members will see their genotype report below and the solutions in the Lifehacks section. Consider joining today.CCR5 gene, immune response
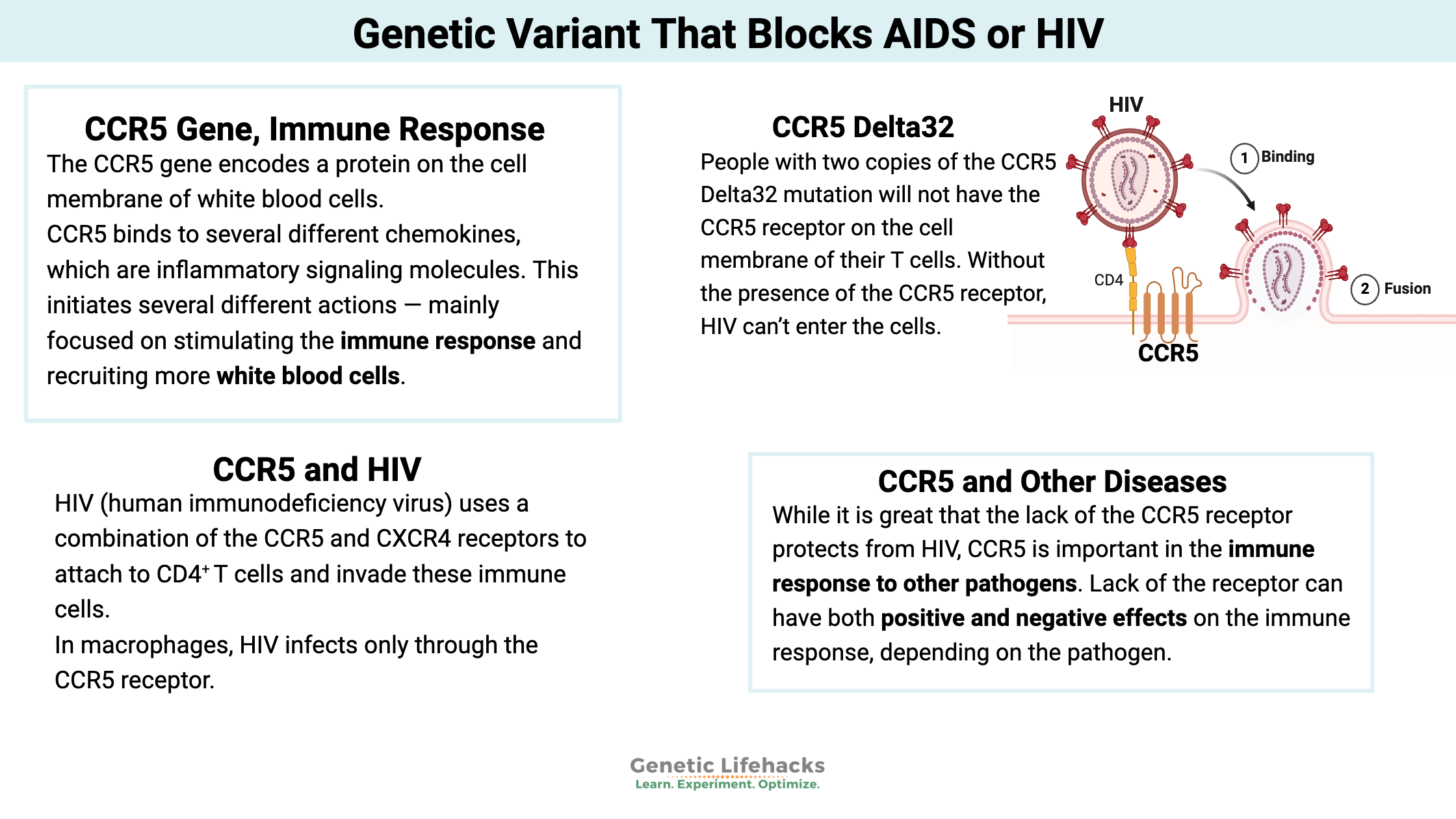
The CCR5 (chemokine receptor type 5) gene encodes a protein on the cell membrane of white blood cells, specifically T cells, macrophages, dendritic cells, eosinophils, and microglial cells. It is a part of your immune system’s response to foreign invaders, and it is also an essential part of how HIV can hijack immune cells.
As a receptor on T cells, CCR5 binds to several different chemokines, which are inflammatory signaling molecules. When the chemokine binds to the CCR5 receptor, it initiates several different actions — mainly focused on stimulating the immune response and recruiting more white blood cells to the area.[ref]
T cells, specifically CD4+ T cells, are an important part of the body’s immune defense system. T cells activate B cells to respond to viruses. Low T cell counts increase susceptibility to pathogens.[ref]
How HIV infects a cell:
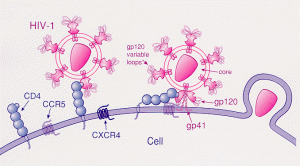
HIV (human immunodeficiency virus) uses a combination of the CCR5 and CXCR4 receptors to attach to CD4+ T cells and invade these immune cells. There are two subtypes of HIV, called HIV-1 and HIV-2. HIV-1 is the more common type, with high infectivity and high virulence.
HIV is an enveloped retrovirus, meaning its viral RNA is integrated into the host cell’s nuclear DNA. Once the virus integrates into the host’s T cell DNA, it may become latent (and remain undetected) for a period of time. Alternatively, the integrated viral genes can be transcribed, and new virus particles can be packaged up and released from the cell at a very fast pace. These newly replicated and released viruses then infect other T cells, continuing the infection process.
HIV infects CD4+ T cells by attaching a spike protein (gp120 glycoprotein) to the CCR5 and CXCR4 receptors. In macrophages, HIV infects only through the CCR5 receptor.[ref]
The infected T cells can replicate the virus in huge numbers. But HIV also triggers abnormal priming of the T cells, resulting in cell death via apoptosis.[ref]
The fact that HIV can lie dormant in T cells is important. T memory cells, infected with the latent virus, persist, and the virus lingers in the body for a lifetime. Other viruses, such as members of the Herpes family of viruses, can also persist in a latent state. For example, the varicella-zoster virus (part of the Herpes family) causes chickenpox when first infected and then lies dormant in the body, only to re-emerge as shingles later in life.[ref]
AIDS from HIV:
Acquired Immunodeficiency Syndrome (AIDS) is caused by the HIV virus. It is a condition in which the immune system is seriously damaged. Over time, the rate of T-cell apoptosis becomes greater than the body’s ability to replace the cells. This shift results in not enough CD4+ T cells left to fight off infections.
To be diagnosed with AIDS, a person’s CD4+ T cell level needs to drop to a certain point. The decrease in T cells allows opportunistic infections to take hold, sometimes leading to death.[ref]
CCR5 Delta32 mutation: resistance to HIV
In the mid-90s, geneticists investigated why some people who were exposed to HIV either didn’t get HIV at all or didn’t progress to AIDS. They found a mutation in the CCR5 gene, called the Delta32 mutation, that prevented the HIV virus from binding to T cells and macrophages.[ref]
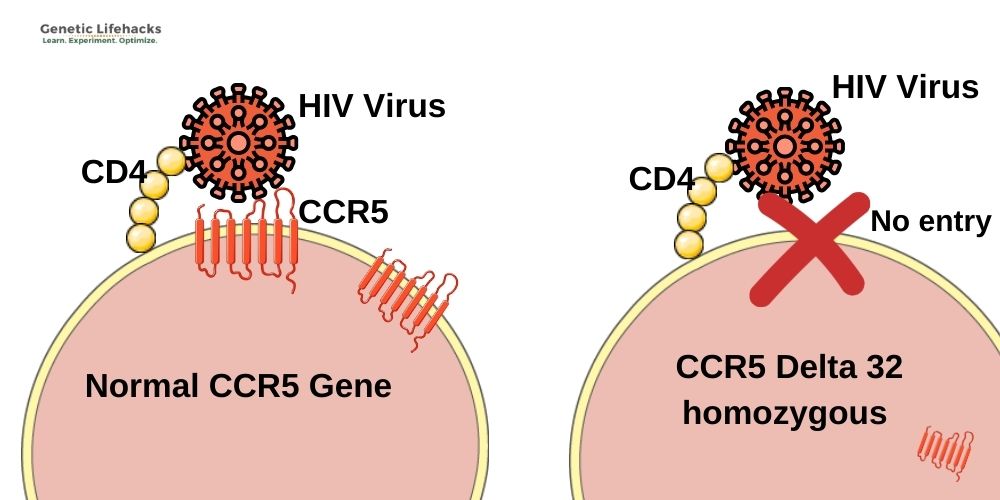
The CCR5 Delta32 mutation causes the protein to be truncated and not moved to the cell surface as a receptor. People with two copies of the mutation will not have the CCR5 receptor on the cell membrane of their T cells. Without the presence of the CCR5 receptor, HIV can’t enter the cells. As one study puts it: “Therefore, homozygous individuals for this polymorphism (Δ32/Δ32) show virtually total protection against HIV type 1 infection, since no CCR5 expression is verified on the cell surface (no HIV−CCR5 interaction at the cell surface is possible)”.[ref]
The first person in the world known to be cured of HIV, the “Berlin Patient“, was Timothy Ray Brown, an American living in Berlin. While HIV-positive, he contracted leukemia, and in 2007 he was given a bone marrow transplant from a donor with two copies of the CCR5 Delta32 (CCR5-Δ32) mutation. After three months, he no longer had detectable HIV in his blood.
Since the ‘Berlin Patient’, several more people have been cured of HIV through bone marrow transplants. Recently, a woman with AIDS and leukemia received an umbilical cord blood treatment from someone with the CCR5 mutation, along with cells from a relative who matched her bone marrow. The woman received the transplant in 2017 and discontinued antiretroviral therapy a few years later. Over a year after discontinuing antiretroviral therapy, her blood work still shows no sign of HIV.[ref]
Resistance to HIV and AIDS:
- People who are Delta32 homozygous (two copies of the variant) have a significantly reduced chance of getting HIV when exposed. It isn’t complete protection for everyone (depends a bit on the strain of HIV), but most people with two copies of the Delta32 variant will not get HIV when exposed.[ref][ref][ref]
- People who carry one copy of the variant (heterozygous) can still contract HIV. But, they have a decreased risk of progression from HIV to AIDs and a significantly reduced risk of death from AIDS.[ref][ref]
Common sense note: While statistically, the odds of getting HIV with two copies of the CCR5-Δ32 are really low, this is not something I would test out. Even if you carry this variant, don’t be stupid and risk an HIV infection. HIV mutates a lot, and the Delta32 variant may not protect against different strains.[ref]
CCR5 and other diseases:
While it is great that the lack of the CCR5 receptor protects from HIV, CCR5 is important in the immune response to other pathogens. Lack of the receptor can have both positive and negative effects on the immune response, depending on the pathogen.
Here are some of the recent studies on the CCR5-Δ32 variant (rs333):
- A preliminary (small) study found that carrying two copies of the Delta32 variant may make the hepatitis B vaccine less effective.[ref]
- A small study shows that the Delta32 mutation may be protective against Crimean-Congo hemorrhagic fever.[ref]
- Carriers of the CCR5-Δ32 variant fare worse with West Nile Virus.[ref][ref]
- Caucasian carriers of Delta32 may have a reduced risk of atherosclerosis.[ref] Asian carriers, though, may be at an increased risk for atherosclerosis.[ref]
- Depression is a common complication after having a stroke. Carriers of the CCR5-Δ32 variant are less likely to develop depression after a stroke.[ref]
- Carriers of the Delta32 variant had a higher mortality rate and severity rate with the swine flu (H1N1).[ref][ref]
A recent study of patients with COVID-19 found that those with the CCR5-Δ32 variant were less likely to be hospitalized with severe symptoms.[ref]
History of the mutation:
Mutations that become common in the human genome are often due to selection pressure. For example, a mutation that is advantageous and keeps you from dying (of a disease or not bleeding to death) passes down to offspring simply because the person with the mutation is more likely to survive and reproduce.
The Delta32 (Δ32) variant is well studied on various immune system topics. It is thought that the mutation first arose in Northern Europe and was preferentially passed on in Caucasian populations due to increasing resistance to smallpox.[ref] Others theorize that the mutation has been in the Caucasian population for about a millennium, with some researchers tying the frequency to likely resistance to the bubonic plague.[ref][ref][ref]
The highest frequency of Delta32 variants is found in the Faroe Islands (Denmark), with 2.3% of the population being homozygous (2 copies) for the mutation. In general, about 10% of people with European ancestry carry one copy of the CCR5-Δ32 variant, with around 1% having two copies. The prevalence is much lower in Asian and African population groups. For example, the frequency of one copy of the variant is about 4% in Nigeria but only around 0.4% in China.[ref][ref]
CCR5 Delta32 HIV Mutation Genotype Report:
Related Articles and Topics:
References:
About HIV/AIDS | HIV Basics | HIV/AIDS | CDC. 1 June 2021, https://www.cdc.gov/hiv/basics/whatishiv.html.
André, Sonia, et al. “T Cell Apoptosis Characterizes Severe Covid-19 Disease.” Cell Death & Differentiation, Jan. 2022, pp. 1–14. www.nature.com, https://doi.org/10.1038/s41418-022-00936-x.
Bigham, Abigail W., et al. “Host Genetic Risk Factors for West Nile Virus Infection and Disease Progression.” PLoS ONE, vol. 6, no. 9, 2011. www.ncbi.nlm.nih.gov, https://doi.org/10.1371/journal.pone.0024745.
Blanpain, Cédric, et al. “CCR5 and HIV Infection.” Receptors & Channels, vol. 8, no. 1, 2002, pp. 19–31.
Bouwman, Abigail, et al. “Ancient DNA Investigation of a Medieval German Cemetery Confirms Long-Term Stability of CCR5-Δ32 Allele Frequencies in Central Europe.” Human Biology, vol. 89, no. 2, Apr. 2017, pp. 119–24. PubMed, https://doi.org/10.13110/humanbiology.89.2.02.
Brown, Timothy Ray. “I Am the Berlin Patient: A Personal Reflection.” AIDS Research and Human Retroviruses, vol. 31, no. 1, Jan. 2015, pp. 2–3. PubMed Central, https://doi.org/10.1089/aid.2014.0224.
Cuesta-Llavona, Elías, et al. “Variant-Genetic and Transcript-Expression Analysis Showed a Role for the Chemokine-Receptor CCR5 in COVID-19 Severity.” International Immunopharmacology, vol. 98, Sept. 2021, p. 107825. www.ncbi.nlm.nih.gov, https://doi.org/10.1016/j.intimp.2021.107825.
Cyranoski, David. “First CRISPR Babies: Six Questions That Remain.” Nature, Nov. 2018. www.nature.com, https://doi.org/10.1038/d41586-018-07607-3.
Ellwanger, Joel Henrique, Bruna Kulmann-Leal, et al. “Beyond HIV Infection: Neglected and Varied Impacts of CCR5 and CCR5Δ32 on Viral Diseases.” Virus Research, vol. 286, Sept. 2020, p. 198040. www.ncbi.nlm.nih.gov, https://doi.org/10.1016/j.virusres.2020.198040.
Ellwanger, Joel Henrique, Valéria de Lima Kaminski, et al. “CCR5 and CCR5Δ32 in Bacterial and Parasitic Infections: Thinking Chemokine Receptors Outside the HIV Box.” International Journal of Immunogenetics, vol. 47, no. 3, June 2020, pp. 261–85. PubMed, https://doi.org/10.1111/iji.12485.
Falcon, A., et al. “CCR5 Deficiency Predisposes to Fatal Outcome in Influenza Virus Infection.” The Journal of General Virology, vol. 96, no. 8, Aug. 2015, pp. 2074–78. PubMed, https://doi.org/10.1099/vir.0.000165.
Galvani, Alison P., and Montgomery Slatkin. “Evaluating Plague and Smallpox as Historical Selective Pressures for the CCR5-Δ32 HIV-Resistance Allele.” Proceedings of the National Academy of Sciences, vol. 100, no. 25, Dec. 2003, pp. 15276–79. www.pnas.org, https://doi.org/10.1073/pnas.2435085100.
Ganczak, Maria, et al. “Possible Impact of 190G > A CCR2 and Δ32 CCR5 Mutations on Decrease of the HBV Vaccine Immunogenicity—A Preliminary Report.” International Journal of Environmental Research and Public Health, vol. 14, no. 2, Feb. 2017, p. 166. PubMed Central, https://doi.org/10.3390/ijerph14020166.
Huang, Y., et al. “The Role of a Mutant CCR5 Allele in HIV-1 Transmission and Disease Progression.” Nature Medicine, vol. 2, no. 11, Nov. 1996, pp. 1240–43. PubMed, https://doi.org/10.1038/nm1196-1240.
Hummel, S., et al. “Detection of the CCR5-Delta32 HIV Resistance Gene in Bronze Age Skeletons.” Genes and Immunity, vol. 6, no. 4, June 2005, pp. 371–74. PubMed, https://doi.org/10.1038/sj.gene.6364172.
Jones, K. L., et al. “Chemokine Receptor CCR5: From AIDS to Atherosclerosis.” British Journal of Pharmacology, vol. 162, no. 7, Apr. 2011, pp. 1453–69. PubMed, https://doi.org/10.1111/j.1476-5381.2010.01147.x.
Keynan, Yoav, et al. “Chemokine Receptor 5 Δ32 Allele in Patients with Severe Pandemic (H1N1) 2009.” Emerging Infectious Diseases, vol. 16, no. 10, Oct. 2010, p. 1621. www.ncbi.nlm.nih.gov, https://doi.org/10.3201/eid1610.100108.
Kolata, Gina, and Pam Belluck. “Why Are Scientists So Upset About the First Crispr Babies?” The New York Times, 5 Dec. 2018. NYTimes.com, https://www.nytimes.com/2018/12/05/health/crispr-gene-editing-embryos.html.
Lim, Jean K., et al. “Genetic Deficiency of Chemokine Receptor CCR5 Is a Strong Risk Factor for Symptomatic West Nile Virus Infection: A Meta-Analysis of 4 Cohorts in the US Epidemic.” The Journal of Infectious Diseases, vol. 197, no. 2, Jan. 2008, pp. 262–65. PubMed, https://doi.org/10.1086/524691.
Liu, Rong, et al. “Homozygous Defect in HIV-1 Coreceptor Accounts for Resistance of Some Multiply-Exposed Individuals to HIV-1 Infection.” Cell, vol. 86, no. 3, Aug. 1996, pp. 367–77. www.cell.com, https://doi.org/10.1016/S0092-8674(00)80110-5.
Mandavilli, Apoorva. “A Woman Is Cured of H.I.V. Using a Novel Treatment.” The New York Times, 15 Feb. 2022. NYTimes.com, https://www.nytimes.com/2022/02/15/health/hiv-cure-cord-blood.html.
Parczewski, Milosz, et al. “Risk of All-Cause Mortality in HIV Infected Patients Is Associated with Clinical, Immunologic Predictors and the CCR5 Δ32 Deletion.” PLoS ONE, vol. 6, no. 7, July 2011, p. e22215. PubMed Central, https://doi.org/10.1371/journal.pone.0022215.
Ruiz-Mateos, Ezequiel, et al. “Association of Heterozygous CCR5Δ32 Deletion with Survival in HIV-Infection: A Cohort Study.” Antiviral Research, vol. 150, Feb. 2018, pp. 15–19. PubMed, https://doi.org/10.1016/j.antiviral.2017.12.002.
Rustemoglu, Aydin, et al. “The Possible Role of CCR5Δ32 Mutation in Crimean-Congo Hemorrhagic Fever Infection.” Journal of Medical Virology, vol. 89, no. 10, Oct. 2017, pp. 1714–19. PubMed, https://doi.org/10.1002/jmv.24865.
Siliciano, Robert F., and Warner C. Greene. “HIV Latency.” Cold Spring Harbor Perspectives in Medicine:, vol. 1, no. 1, Sept. 2011. www.ncbi.nlm.nih.gov, https://doi.org/10.1101/cshperspect.a007096.
Solloch, Ute V., et al. “Frequencies of Gene Variant CCR5-Δ32 in 87 Countries Based on next-Generation Sequencing of 1.3 Million Individuals Sampled from 3 National DKMS Donor Centers.” Human Immunology, vol. 78, no. 11–12, Nov. 2017, pp. 710–17. PubMed, https://doi.org/10.1016/j.humimm.2017.10.001.
Tene, Oren, et al. “CCR5-Δ32 Polymorphism: A Possible Protective Factor for Post-Stroke Depressive Symptoms.” Journal of Psychiatry & Neuroscience : JPN, vol. 46, no. 4, Aug. 2021, p. E431. www.ncbi.nlm.nih.gov, https://doi.org/10.1503/jpn.200197.
Tuen, Michael, et al. “Immune Correlates of Disease Progression in Linked HIV-1 Infection.” Frontiers in Immunology, vol. 10, 2019. www.ncbi.nlm.nih.gov, https://doi.org/10.3389/fimmu.2019.01062.
Wilen, Craig B., et al. “HIV: Cell Binding and Entry.” Cold Spring Harbor Perspectives in Medicine, vol. 2, no. 8, Aug. 2012. www.ncbi.nlm.nih.gov, https://doi.org/10.1101/cshperspect.a006866.
Zhang, Zhongwen, et al. “Association between Chemokine Receptor 5 (CCR5) Delta32 Gene Variant and Atherosclerosis: A Meta-Analysis of 13 Studies.” International Journal of Clinical and Experimental Medicine, vol. 8, no. 1, Jan. 2015, pp. 658–65. PubMed Central, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4358496/.
Debbie Moon is the founder of Genetic Lifehacks. Fascinated by the connections between genes, diet, and health, her goal is to help you understand how to apply genetics to your diet and lifestyle decisions. Debbie has a BS in engineering from Colorado School of Mines and an MSc in biological sciences from Clemson University. Debbie combines an engineering mindset with a biological systems approach to help you understand how genetic differences impact your optimal health.