Key takeaways:
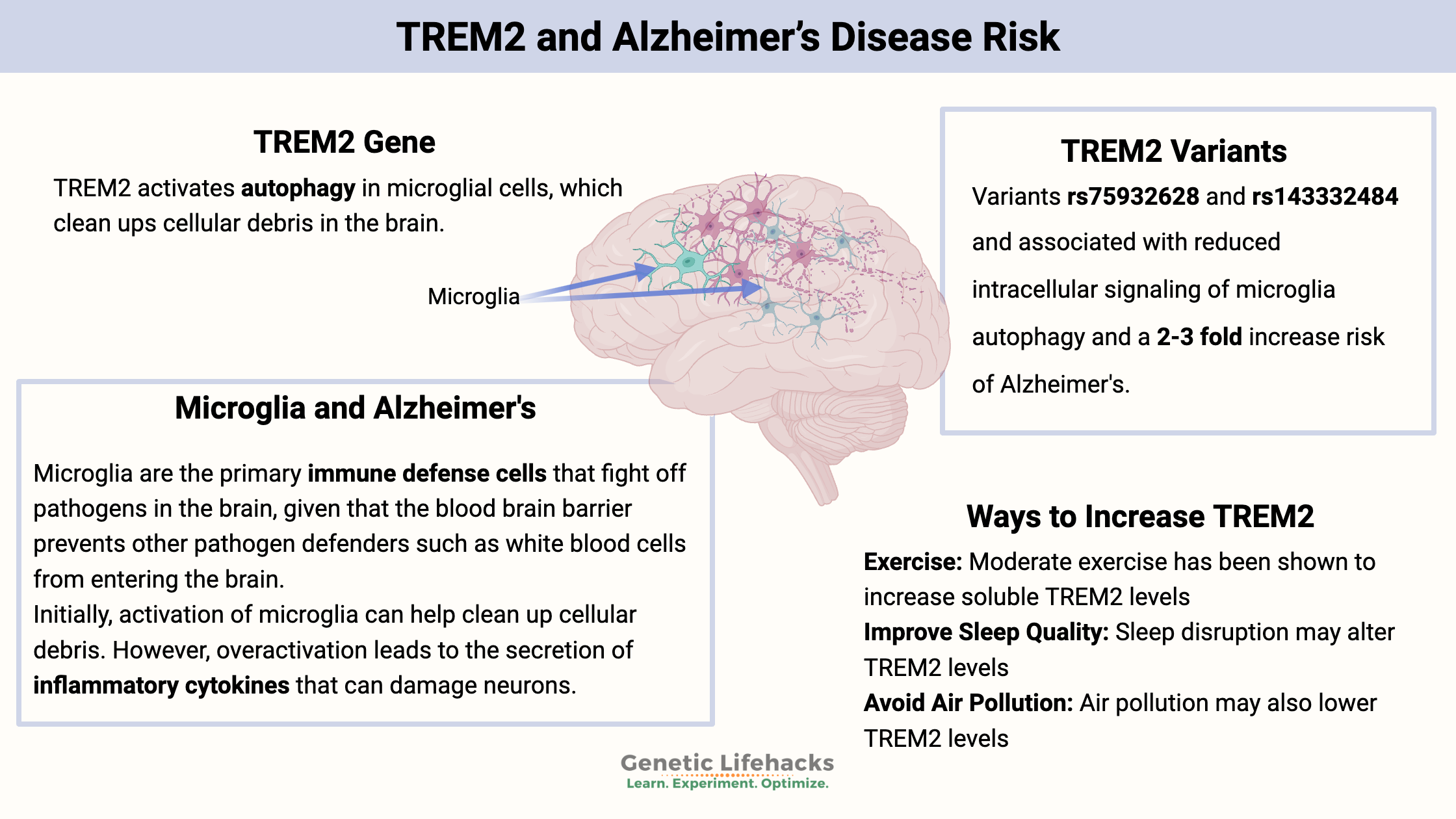
~ TREM2 activates autophagy in microglial cells, which clean ups cellular debris in the brain.
~ Genetic variants in TREM2 cause increased Alzheimer’s risk.
~ Understanding your risk of Alzheimer’s disease can help you prioritize your best ways of preventing Alzheimer’s.
TREM2, Microglia, and Alzheimer’s risk:
There are two types of Alzheimer’s:
- Early-onset, familial Alzheimer’s disease is caused by rare mutations in the APP, PSEN1, and PSEN2 genes.
- A late-onset form of dementia is associated with several genetic variants, including the APOE gene and the TREM2 gene. If you want to know your APOE genotype, you can check it out here.
Genetics research over the past decade has highlighted the importance of the immune response as central to Alzheimer’s disease. Most genetic risk factors discovered for Alzheimer’s involve the brain’s immune system.[ref]
Microglia: brain immune cells
Microglia cells are an important cell type in the brain and spinal cord. Microglia are the primary immune defense cells that fight off pathogens in the brain. But they are also critical for responding to cellular stress in the brain’s neurons and cleaning up cellular debris.
The blood-brain barrier does a good job of keeping most bacteria out of the brain. In turn, the blood-brain barrier also blocks the rest of the body’s immune cells, such as white blood cells, from entering the brain. Thus, microglia are the brain’s resident immune cells. When activated, microglia release cytokines.
Within different regions of the brain, up to 16% of the cells may be microglia. Microglia respond to and interact with other molecules in their environment, such as pathogen-associated molecules (PAMPs) and cell-damaging molecules in the brain (DAMPs).[ref]
Like many immune system responses, microglial activation can be a double-edged sword. Initially, activation of microglia can help clean up cellular debris. However, overactivation leads to the secretion of inflammatory cytokines that can damage neurons.[ref]
The TREM2 gene and Alzheimer’s
The TREM2 gene encodes a receptor on microglia that receives signals from nearby neurons and then activates several important functions of microglia. For example, TREM2 is essential for activating autophagy or phagocytosis in microglial cells, which is necessary to clear cellular debris in the brain.[ref]
TREM2 can be activated by several different molecules, including APOE, bacterial lipopolysaccharides, and amyloid-β. So you can see how it is at an integral crossroads in how the brain responds to several important molecules in Alzheimer’s pathogenesis.
TREM2 Genotype Report:
Several genetic variants have links to an increased risk for Alzheimer’s disease. The R47H variant has been linked in numerous studies to Alzheimer’s. Study results show that the increase in relative risk of Alzheimer’s disease is 2-3 fold.[ref]
Members: Log in to see your data below.
Not a member? Join here.
Why is this section is now only for members? Here’s why…
Lifehacks:
Diet, exercise, and a healthy lifestyle are all important in preventing Alzheimer’s disease. Seriously- I know it sounds like just the same old general advice, but research studies do show that all of the lifestyle factors do matter in Alzheimer’s disease.
Binge drinking and the oral microbiome:
A new study shows that binge drinking causes a shift in the oral microbiome. Excessive ethanol affects the oral microbiome and couples with increased permeability to the blood-brain barrier, possibly increasing the ability of bacteria to reach the brain.[ref]
Learn more about the research on the oral microbiome and Alzheimer’s.
Related Articles and Topics:
TNF-Alpha: Higher innate levels of this inflammatory cytokine
Do you feel like you are always dealing with inflammation? Joint pain, food sensitivity, etc.? Perhaps you are genetically geared towards a higher inflammatory response. Tumor necrosis factor (TNF) is an inflammatory cytokine that acts as a signaling molecule in our immune system.
Rapamycin, mTOR, and Your Genes
Rapamycin is an antibiotic used as an immunosuppressant, an anti-cancer agent, and to prevent blocked arteries. Rapamycin is now the focus of longevity and healthspan-extending research through its inhibition of mTOR.
Boosting NAD+ levels to fight the diseases of aging
Explore the research about how nicotinamide riboside (NR) and NMN are being used to reverse aging. Learn about how your genes naturally affect your NAD+ levels and how this interacts with the aging process.
Melatonin: Key to Health and Longevity
More than just a sleep hormone, melatonin is at the heart of many health topics. Your genetic variants play a big role in the production of melatonin. Learn how your lifestyle and diet interact with your melatonin-related genes.
References:
Bachiller, Sara, et al. “Microglia in Neurological Diseases: A Road Map to Brain-Disease Dependent-Inflammatory Response.” Frontiers in Cellular Neuroscience, vol. 12, 2018. Frontiers, https://www.frontiersin.org/article/10.3389/fncel.2018.00488.
Gray, Sophie C., et al. “Shifting Equilibriums in Alzheimer’s Disease: The Complex Roles of Microglia in Neuroinflammation, Neuronal Survival and Neurogenesis.” Neural Regeneration Research, vol. 15, no. 7, Jan. 2020, pp. 1208–19. PubMed Central, https://doi.org/10.4103/1673-5374.272571.
Greve, Hendrik J., et al. “Diesel Exhaust Impairs TREM2 to Dysregulate Neuroinflammation.” Journal of Neuroinflammation, vol. 17, no. 1, Nov. 2020, p. 351. PubMed, https://doi.org/10.1186/s12974-020-02017-7.
Guerreiro, Rita, et al. “TREM2 Variants in Alzheimer’s Disease.” The New England Journal of Medicine, vol. 368, no. 2, Jan. 2013, pp. 117–27. PubMed, https://doi.org/10.1056/NEJMoa1211851.
Hall-Roberts, Hazel, et al. “TREM2 Alzheimer’s Variant R47H Causes Similar Transcriptional Dysregulation to Knockout, yet Only Subtle Functional Phenotypes in Human IPSC-Derived Macrophages.” Alzheimer’s Research & Therapy, vol. 12, Nov. 2020, p. 151. PubMed Central, https://doi.org/10.1186/s13195-020-00709-z.
Jay, Taylor R., et al. “TREM2 in Neurodegenerative Diseases.” Molecular Neurodegeneration, vol. 12, Aug. 2017, p. 56. PubMed Central, https://doi.org/10.1186/s13024-017-0197-5.
Jensen, Camilla Steen, et al. “Exercise as a Potential Modulator of Inflammation in Patients with Alzheimer’s Disease Measured in Cerebrospinal Fluid and Plasma.” Experimental Gerontology, vol. 121, July 2019, pp. 91–98. PubMed, https://doi.org/10.1016/j.exger.2019.04.003.
Ren, Siqiang, et al. “TNF-α-Mediated Reduction in Inhibitory Neurotransmission Precedes Sporadic Alzheimer’s Disease Pathology in Young Trem2R47H Rats.” The Journal of Biological Chemistry, vol. 296, June 2021, p. 100089. PubMed, https://doi.org/10.1074/jbc.RA120.016395.
Sims, Rebecca, et al. “Rare Coding Variants in PLCG2, ABI3 and TREM2 Implicate Microglial-Mediated Innate Immunity in Alzheimer’s Disease.” Nature Genetics, vol. 49, no. 9, Sept. 2017, pp. 1373–84. PubMed Central, https://doi.org/10.1038/ng.3916.
Tapp, Zoe M., et al. “Sleep Disruption Exacerbates and Prolongs the Inflammatory Response to Traumatic Brain Injury.” Journal of Neurotrauma, vol. 37, no. 16, Aug. 2020, pp. 1829–43. PubMed, https://doi.org/10.1089/neu.2020.7010.
Wang, Shoutang, et al. “Anti-Human TREM2 Induces Microglia Proliferation and Reduces Pathology in an Alzheimer’s Disease Model.” The Journal of Experimental Medicine, vol. 217, no. 9, Sept. 2020, p. e20200785. PubMed, https://doi.org/10.1084/jem.20200785.
Yussof, Ayuni, et al. “A Meta-Analysis of the Effect of Binge Drinking on the Oral Microbiome and Its Relation to Alzheimer’s Disease.” Scientific Reports, vol. 10, no. 1, Nov. 2020, p. 19872. www.nature.com, https://doi.org/10.1038/s41598-020-76784-x.
Zhong, Li, and Xiao-Fen Chen. “The Emerging Roles and Therapeutic Potential of Soluble TREM2 in Alzheimer’s Disease.” Frontiers in Aging Neuroscience, vol. 11, 2019. Frontiers, https://www.frontiersin.org/article/10.3389/fnagi.2019.00328.