When it comes to chronic diseases, the “Big 3” are heart disease, cancer, and type 2 diabetes. Heart disease is the number one killer in the US, with cancer a close second. Diabetes numbers are increasing at an alarming rate.
Genetically, one gene connects all three of these chronic diseases. BMAL1 is one of the core circadian clock genes, and lifestyle factors, along with genetic variants, are important here. We will dive into the science of circadian rhythm to understand why these genetic variants are important for health and longevity. Members will see their genotype report below, plus additional solutions in the Lifehacks section. Join today.
BMAL1 Gene: Circadian clock, diabetes, heart disease, and cancer
Our circadian rhythm is driven by two pairs of proteins acting together like a molecular clock. These two pairs of proteins rise and fall at different times to set our 24-hour daily rhythm.
One pair of proteins, CLOCK and BMAL1, influence biological activities during the daytime.
Another pair governs the night half of the rhythm (PER and CRY).
During the day, BMAL1 and CLOCK levels rise; at night, PER’s and CRY’s levels peak. This molecular clock sets our whole body’s circadian rhythm.
Circadian rhythm biology is not just unique to humans – plants, all animals, and even some bacteria have similar circadian genes. It makes sense that your body can turn on processes that are needed when you are active (e.g., digestive enzymes, immune system genes) and then turn them off at night.
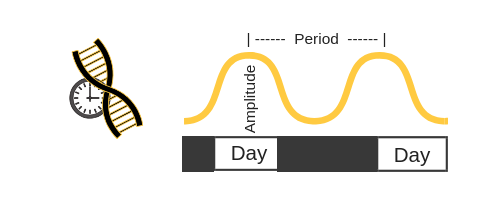
When it comes to the circadian clock genes, researchers like to use terminology like ‘period’ and ‘amplitude’. Let’s take BMAL1 as an example: the levels will rise and then fall (amplitude) over the course of 24 hours (period).
It is easier to picture this if you visualize the amount of BMAL1 rising and falling like a sine wave:
Quite a lot of your body’s processes rise and fall over the course of the day. In fact, researchers estimate that 20 – 43% of genes are following a circadian pattern.
These circadian rhythms are obvious when you think about our natural sleep/wake cycle. Other biological rhythms include cortisol levels, body temperature, and blood pressure.
Blood pressure and BMAL1:
Blood pressure also follows a natural rhythm over the course of a 24-hour day, dipping by about 10% at night.[ref] Dipping at night is important, and people with high blood pressure often lose that oscillation.
- Animal studies show that disrupting the natural dipping pattern causes heart disease.[ref]
- Human studies have found that BMAL1 genetic variants are linked to heart disease.[ref]
- And a recent study found that loss of BMAL1 causes atherosclerosis.[ref]
Heart disease and BMAL1:
A recent animal study sheds some light on why BMAL1 is so important in heart disease. The study found that reducing BMAL1 in heart muscle cells directly affects the mitochondria (the powerhouse of the cell). Without good mitochondrial function, the heart muscle cells can’t function optimally, eventually leading to “compromised heart function and dilated cardiomyopathy”. [ref]
Cancer and BMAL1:
The 24-hour day is based on the sun rising and setting… And light is a driving factor in resetting our circadian rhythm, keeping us on track each day. Electric lights at night, though, can disrupt our circadian rhythm genes.
Both exposure to light at night and working the night shift cause an increased risk of breast cancer. Of course, not everyone working the night shift gets breast cancer. Genetic variants seem to play a role in whether the light at night significantly increases your risk of breast cancer.
BMAL1 gene variants have been tied to both increased[ref][ref] and decreased risk of cancer[ref], depending on the variant and type of cancer.
To find out how BMAL1 influences cancer, researchers investigated what happens when BMAL1 is deleted in a cell. The study found that in some types of cancer cells, deleting BMAL1 increased invasive cancer, but in other cells, it triggered apoptosis which killed the cancer cell.[ref]
Diabetes and BMAL1:
Several circadian genetic variants have been found to increase the risk of type 2 diabetes. Some, like melatonin receptor variants, influence insulin release and lead to insulin resistance for those who eat too late at night.
In the pancreas, cells called beta cells are responsible for creating and secreting insulin needed for shuttling glucose into all of the cells of the body. Without enough beta cells, the pancreas has a hard time producing enough insulin. BMAL1 is involved in pancreatic beta-cell proliferation as well as the regulation of adipogenesis (fat cell creation).[ref]
BMAL1 (ARNTL) Genotype Report:
Lifehacks:
Lifestyle changes:
Circadian optimization is foundational.
- Block out blue light at night either by turning off electronics and bright overhead lights or by wearing blue-blocking glasses.
- Bright light during the day is also essential, so get outside first thing in the morning.
- Eat on a regular schedule during the day, and stop eating at least three hours before bedtime.
Related Articles and Topics:
Circadian Rhythms: Genes at the Core of Our Internal Clocks
Circadian rhythms are the natural biological rhythms that shape our biology. Most people know about the master clock in our brain that keeps us on a wake-sleep cycle over 24 hours. This is driven by our master ‘clock’ genes.
Blue-blocking Glasses: Why? Which ones?
Optimizing circadian rhythm starts by blocking out light in the blue wavelengths at night. Let me convince you with some dazzling science, and then I’ll explain what some of your options are for blue-blocking glasses.