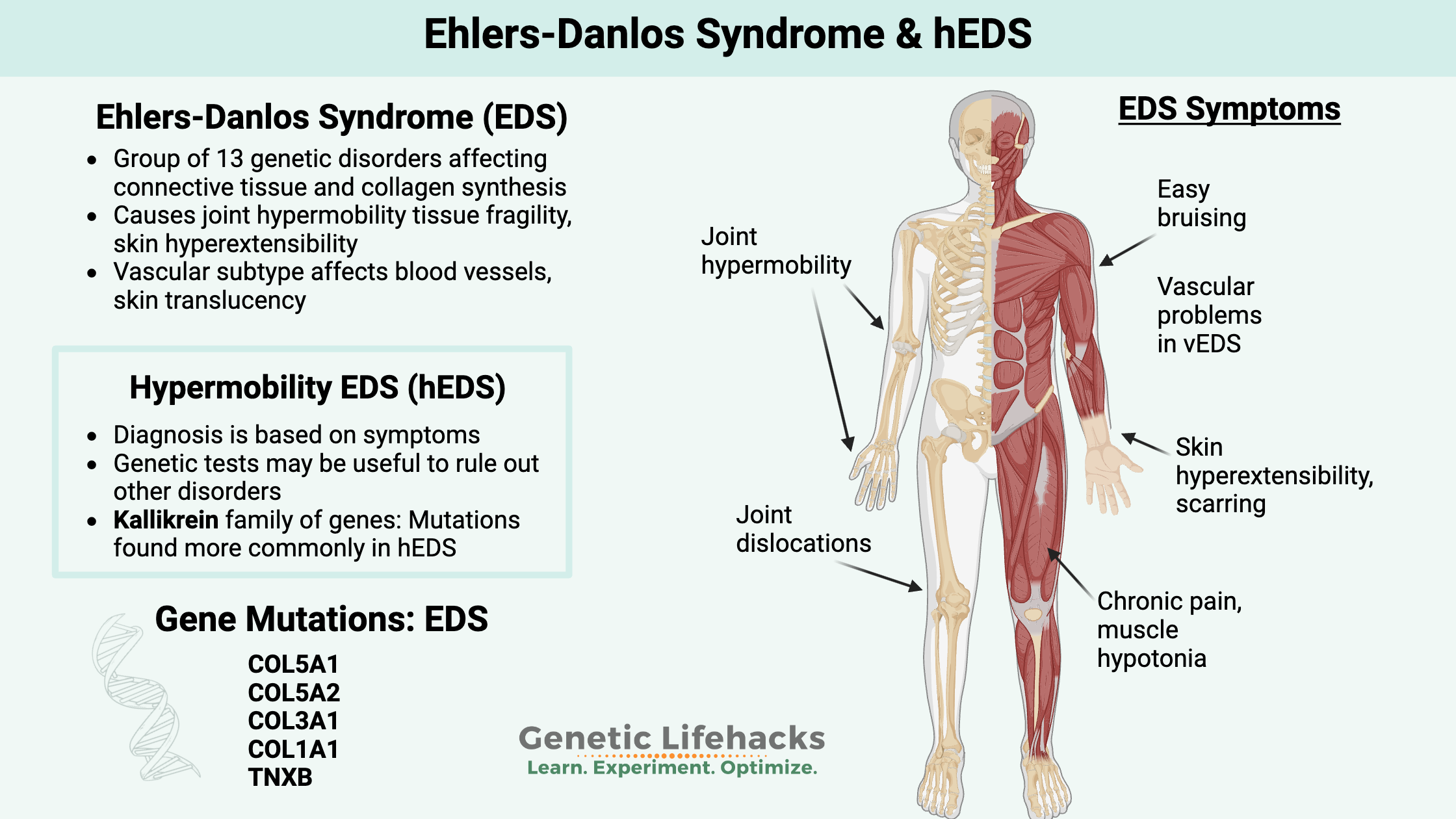
Key takeaways:
~ Ehlers-Danlos Syndromes are a group of disorders of connective tissue, that change the way that collagen forms in the joints, ligaments, and skin.
~ Rare genetic mutation collagen genes cause changes to different connective tissues, giving rise to inherited EDS subtypes. Many (but not all) of these mutations are covered in 23andMe or AncestryDNA data (see the Genotype report).
~ Hypermobility EDS is diagnosed based on symptoms, and genetic tests may be used to rule out other connective tissue disorders.
This article explores the research on Ehlers-Danlos syndrome and describes the genetic mutations that cause some of the disorder’s subtypes. You can learn more about how collagen disorders affect people and check your genetic data (23andMe v5 data works best).
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Consider joining today.
Ehlers-Danlos Syndromes (EDS): inherited connective tissue disorders and your genes
Ehlers-Danlos Syndromes (EDS) are a group of genetic disorders impacting the connective tissues in the body.
Edvard Ehlers and Henri Danlos, two dermatologists, initially described Ehlers-Danlos in the early 1900s. It was formally identified and classified in the 1940s.
In 2017, the Ehlers-Danlos Syndrome International Consortium categorized 13 different subtypes and the criteria for diagnosis. Previously, the Berlin and Villefranche nosologies were used to describe several subtypes of EDS.
The different EDS subtypes are defined by the genetic mutation the person carries — with one exception, hypermobility EDS is diagnosed without any genetic testing.
Below are the different EDS subtypes. (Table adapted from PMC7786113)
| Subtypes | Notable features | Gene(s) involved | Structural effect of disorder |
|---|---|---|---|
| Classical EDS | Skin fragility, hyperextensibility Atrophic scarring Joint hypermobility Common joint dislocations Easy bruising Hernia |
COL5A1 COL5A2 Rarely COL1A1 |
Affects primary structure and processing of collagen |
| Classical-like EDS | Skin fragility, hyperextensibility Joint hypermobility Common joint dislocations Easy bruising Foot deformities Peripheral edema Mild muscle weakness and atrophy Polyneuropathy Organ prolapse |
TNXB | Affects myomatrix structure and function |
| Cardiac-valvular EDS | Joint hypermobility Skin hyperextensibility Severe defects of cardiac valves Hernia Pectus deformity |
COL1A2 | Affects primary structure and processing of collagen |
| Vascular EDS | Aneurysm, dissection, or rupture of arteries Perforation or rupture of gastrointestinal organs Rupture of the uterus during pregnancy Skin translucency Easy bruising Distinctive facial features |
COL3A1 Rarely COL1A1 |
Affects primary structure and processing of collagen |
| Hypermobile EDS | Joint hypermobility Velvety/soft skin Slightly hyperextensible skin Common joint dislocations and subluxations Chronic pain |
Causative gene unidentified in most cases | |
| Arthrochalasia EDS | Severe joint hypermobility at birth Congenital dislocation of bilateral hips Skin hyperextensibility Atrophic scarring |
COL1A1 COL1A2 |
Affects primary structure and processing of collagen |
| Dermatosparaxis EDS | Extreme skin fragility and laxity Delayed closure of fontanelle Distinctive facial features Blue discoloration of the sclera Short fingers and stature |
ADAMTS2 | Affects primary structure and processing of collagen |
| Kyphoscoliotic EDS | Kyphoscoliosis Joint hyperflexibility Muscle hypotonia Ocular fragility Skin hyperextensibility Atrophic scarring Arterial rupture Respiratory compromise in severe cases |
PLOD1 FKBP14 |
Affects folding and cross-linking of collagen |
| Brittle cornea Syndrome | Fragile cornea with risk of rupture Blue sclerae Early keratoconus and keratoglobus Severe myopia Detachment of retina Deafness Mild contractures of fingers Distal joint hypermobility |
ZNF469 PRDM5 |
Affects intracellular processes |
| Spondylodysplastic EDS | Hypotonia of muscles Short stature and bowing of limbs Skin hyperextensibility Delayed cognitive and motor development |
B4GALT7 B3GALT6 SLC39A13 |
Affects biosynthesis of glycosaminoglycan and intracellular processes |
| Musculocontractural EDS | Multiple contractures Club foot Early craniofacial abnormalities Skin hyperextensibility Increased wrinkling of palms Kidney stones |
CHST14 DSE |
Affects biosynthesis of glycosaminoglycans |
| Myopathic EDS | Congenital muscle weakness and atrophy, lessening with age Contractures of proximal joints Joint hypermobility Developmental motor delay |
COL12A1 | Affects myomatrix structure and function |
| Periodontal EDS | Early, severe periodontitis Detachment of gingiva Pretibial plaques Easy bruising Joint hypermobility Higher risk of infection |
C1R, C1S | Affects complement pathways |
Collagen and connective tissue: Causes of EDS
EDS is a connective tissue disorder. Other connective tissue disorders include Marfan syndrome, lupus, rheumatoid arthritis, Churg-Strauss syndrome, and more.[ref]
What is connective tissue? Between the bone, muscles, organs, and nerves is a connective tissue that holds everything together. Connective tissue is made up of collagen and elastic fibers, cells, and the extracellular matrix.
Tendons and ligaments are made up of dense connective tissue, with collagen fibers arranged in parallel for structure. Elastin is also found in the ligaments as well as in the skin.
Collagen is the most abundant protein in the body. The connective tissue cells, especially fibroblasts, secrete collagen into the extracellular matrix, forming a fibrous network.
Multiple types of collagen: The most common type of collagen is type 1, which is found in skin, tendons, blood vessels, and bones. Cartilage, such as in your nose or ears, is mainly type II collagen. Type III collagen is found supporting soft tissues, while type IV makes up part of the epithelium. Finally, type V collagen is important in cell surfaces, hair, and the placenta. The different types of collagen can also interact with each other in forming different types of connective tissue.
The main amino acids that make up the collagen protein include glycine, proline, and alanine.
Synthesizing collagen: Multiple genes encode proteins important in collagen formation. Many of these start with the prefix COL, such as COL5A1. Interacting with the genes are specific enzymes needed in the formation of the connective tissue.
Inside connective tissue cells, a precursor form of collagen, called pro-collagen, is assembled. Then it is secreted into the extracellular space, and the final collagen fiber is assembled with the help of enzymes known as collagen peptidases.
The COL genes encode the proteins that form the procollagen, which is then acted upon by enzymes to create the different types of collagen in the body.
Focusing on COL5A1 and COL5A2:
Most people diagnosed with classical Ehlers-Danlos syndrome (type III) have a single mutation in the COL5A1 or COL5A2 gene.
The COL5A1 and COL5A2 genes encode proteins important in the formation of Type V collagen. Type I collagen and type V collagen work together to form collagen in certain areas of the body. A complete lack of type V collagen is lethal in embryo formation.[ref]
In wound healing and skin thickness, type V collagen is important. One way that researchers have determined this is through animal studies. Animal models of EDS were created using mice that were bred to lack COL5A1. The lack of COL5A1 causes thin skin along with spontaneous wounds that don’t heal correctly.[ref]
Type V collagen is also important for the joints and spine. Certain mutations in COL5A1 are linked to progressive kyphoscoliosis, which is a curve in the upper back that is both sideways and an arched hump.[ref]
Symptoms of classical Ehlers-Danlos Syndrome:
A recent study involving 75 people with genetic confirmation of classical EDS illustrated the wide range of symptoms associated with EDS. The people in the study all had a mutation in the COL5A1 gene or the COL5A2 gene.
Symptoms reported for classical EDS include[ref]:
-
- joint hypermobility
- skin hyperextensibility
- scarring from poor wound healing
- delayed motor development
- muscle fatigue and cramps
- mild scoliosis
- blood vessel fragility
- rectal prolapse in childhood
- cervical insufficiency during pregnancy
Facial features typical for EDS included epicanthal folds (upper eyelid skin fold), drooping eyelids, prominent eyes, eyes that are closer together than typical, low-set ears, and elongation in the area between nose and mouth.
The key point here is that not everyone with a classical EDS mutation will have the same features and symptoms. There is a wide range of phenotypes, depending on the specific mutation as well as other genetic variants and environmental factors. Some people will have more severe symptoms, while others will have milder issues.[ref]
Specific types of Ehlers-Danlos Syndrome:
Classical EDS:
Classic EDS, or EDS type I/II, refers to people with joint hypermobility, skin hyperextensibility, easy bruising, tissue fragility, and scarring. In addition to skin and joint issues, some people with classic EDS will also have vascular (blood vessel) complications.
Genetic mutations in classic EDS:
As discussed above, people with classic EDS have mutations in the COL5A1 or COL5A2 gene. A partial list of mutations in these genes is below in the Genotype report section.
According to research studies, classical EDS is found in about 1 in 20,000 people.[ref]
Vascular Ehlers-Danlos Syndrome:
Considered a rare and severe disorder, vascular Ehlers-Danlos is characterized by fragile, thin skin and blood vessels. The endothelial tissue that lines the blood vessels, intestines, and the uterus is prone to easily rupturing.[ref]Gastrointestinal tract complications are possible.
This can result in an aneurysm, intestinal fistula, or bowel ruptures. Usually diagnosed in their 20s or 30s, people with vEDS often have characteristic thin, translucent skin and easy bruising. Diagnosis often follows a major vascular event (e.g., stent, aneurysm), abdominal aneurysm, or a bowel rupture.[ref][ref]
Genetic mutations in vascular EDS:
Diagnosis with vascular Ehlers-Danlos includes a positive genetic test for a pathogenic mutation in the COL3A1 gene. While many rare genetic diseases require two pathogenic mutations for the disease to occur, vascular Ehlers-Danlos can sometimes be caused by carrying just one mutation in the gene.
There are over 500 known mutations in the COL3A1 gene, and the overall frequency of vEDS is 1 in 150,000 people.[ref]
Dermatosparaxis EDS:
Dermatosparaxis EDS, or EDS type VIIC, involves extremely fragile and lax skin. People with this subtype of EDS are often shorter in stature and have a blue discoloration of the whites of their eyes.
Genetic mutations in dermatosparaxis EDS:
Rare mutations in the ADAMS2 and ADAMS12 genes are responsible for dermatosparaxis EDS. These genes are involved in cleaving the collagen proteins at the right spot for them to be activated.[ref]
Periodontal Ehlers-Danlos Syndrome:
Another subtype of Ehlers-Danlos involves the gums and teeth. Periodontitis and tooth-root absorption are hallmarks of this subtype.[ref]
Genetic mutations in periodontal EDS:
The genetic component of periodontal EDS involves immune system genes that code for part of the complement system. People with periodontal EDS have a mutation in the C1R (complement 1 receptor) gene. In addition to severe periodontitis, people with periodontal EDS can also have easy bruising and joint hypermobility.[ref]
Hypermobile Ehlers-Danlos Syndrome (hEDS):
Hypermobility (or Hypermobile) Ehlers-Danlos syndrome (hEDS), or EDS type III in the newer definition, is characterized by laxity in the ligaments. This can cause joint dislocation, joint weakness, joint pain, nerve pain, or muscle pain. Additionally, some people can have an increased risk of early-onset arthritis and osteoporosis.[ref]
According to the NIH Rare Disease website: “It is generally considered the least severe form of Ehlers-Danlos syndrome (EDS) although significant complications can occur.“[ref]
Hypermobile Ehler’s Danlos is currently diagnosed based entirely on symptoms. Hypermobility of joints in hEDS generally occurs and is noticeable in the first few years of life. In other words, hEDS isn’t something that develops as an adult, but instead, the hypermobility aspects are apparent from early childhood.[ref]
With an hEDS diagnosis, genetic testing is used to rule out other connective tissue disorders, including classic EDS mutations, Marfan syndrome, Loeys-Dietz syndrome, osteogenesis imperfecta, and lateral meningocele syndrome.[ref][ref]
Kallikrein mutations identified in hEDS:
The kallikrein family of enzymes breaks apart protein bonds. They are a class of protein-degrading enzymes. The body is constantly making new proteins and breaking down proteins to alter or inactivate them. While we often focus on the creation of proteins from genes, the degradation and recycling of proteins are equally important and balance everything out.
Humans have 15 genes in the kallikrein family, and the enzymes interact with the extracellular matrix and connective tissue. Kallikrein also plays a role in blood pressure regulation and immune cell function. Altered kallikrein expression is linked to a number of chronic diseases including Alzheimer’s, asthma, atopic dermatitis, CAD, bipolar, kidney disease, MS, psoriasis, and more.[ref]
A new 2024 preprint study (Gensemer, et. al.) investigated the prevalence of rare variants in families with hEDS. The genetic results pointed the researchers to look at kallikrein. Essentially, the researchers looked at whole exome sequencing for two families with multiple hEDS patients. The researchers discovered a rare mutation in the KLK15 (kallikrein 15) gene. They then used mice with the KLK15 gene knocked out to see if deleting the gene caused symptoms similar to hEDS. The researchers found that the lack of KLK15 caused changes in the tendons of the mice and also caused valve dysfunction in the hearts of the mutant mice.
Taking it another step, the researchers looked at genetic data for 197 hEDS patients and found that about of third of them had a rare mutation in one of the KLK genes.[ref]
While it is exciting to see this type of research into a genetic mechanism for hEDS, I want to caution that this is still a preprint paper and that it needs to be replicated in a larger cohort. Currently, these rare mutations aren’t found in direct-to-consumer genetic tests. However, this is an exciting avenue of research for anyone with hEDS because there is a lot of research going on with drug development targeting kallikrein.[ref]
Interestingly (for hEDS), kallikrein interacts with immune response and blood pressure. This ties into the alterations in blood pressure seen in some people with hEDS – as well as the immune activation and mast cell activation.
Kallikreins can be found in plasma or in tissue. Plasma kallikrein is involved in blood pressure regulation through interaction with bradykinin, and it is one way that plasminogen can be activated to plasmin in forming clots. Tissue kallikreins are also involved in blood pressure via the kidneys and are involved in healthy arteries and heart function. In the immune system, tissue kallikrein (KLK1) increases significantly in the lungs during an influenza infection and modulates inflammation. KLK5 and KLK12 interact with coronavirus infections.[ref][ref][ref][ref]
Related articles: Plasminogen and PAI1, fibrinogen
Hypermobility Spectrum Disorder (HSD):
Similar to hEDS, hypermobility spectrum disorder (HSD) is a term being applied to the group of connective tissue disorders that involve hypermobility in one or more joints, subluxations, and joint dislocations.[ref][ref] Joint hypermobility syndrome is another term given when diagnosing people with joint hypermobility and no defined molecular or genetic basis.[ref]
Prevalence of diagnosis:
In the UK, a recent study found that about 1 in 500 people were diagnosed with either joint hypermobility or Ehlers-Danlos Syndrome. This shows a much greater prevalence than earlier diagnoses of EDS based on genetic testing (1 in 20,000) or even previous prevalence of hEDS (1 in 5,000).[ref]
When you read through the diagnostic criteria for hEDS or hypermobility spectrum disorder, it seems like this is cut-and-dry. Certain symptoms = certain diagnoses. But the reality is that everyone is unique, and not everyone fits into a specific box.[ref]
A recent study looked at the whole genomes of people who had hEDS symptoms but no genetic diagnosis. The researchers found that out of 174 patients, there were 10 with EDS mutations found on the whole genome testing. Additionally, there were multiple other mutations that were likely related to the EDS symptoms in other hEDS patients.[ref] The takeaway from the study is that there is a lot more to learn about how genes affect hEDS symptoms.
Does having flexible joints mean that you have Ehlers Danlos or hEDS?
Up to 25% of kids and teens are considered to have joint hypermobility. Girls are more likely than boys in this tendency, and it is thought to cause no complications in kids. Thus, simply being a flexible teenage girl does not necessarily mean it is EDS… instead, other symptoms need to also be present, such as skin laxity, easy bruising (without another cause), or joint dislocations.[ref]
Along with joint hypermobility, many with hypermobility EDS diagnosis also have other co-morbidities.
Common together: POTS and hEDS
POTS stands for postural orthostatic tachycardia syndrome. It is defined as an increase in heart rate of 30 beats/minute (adults) or 40 beats/minute (kids, teens) when going from laying down to standing up.
There is an overlap between people with POTS and hEDS that is not well explained.
Some estimates show that over 40% of people with hEDS also have POTS, but not all research agrees. Part of the difference in studies is that POTS can be defined in different ways in different clinics. Additionally, much of the data in the studies are self-reported rather than measured in a clinic.[ref]
A recent study at Penn State investigated the link between POTS and hEDS. The study included 91 people with clinically diagnosed POTS. Of those, 31% met the clinical criteria for hEDS. Notably, an additional 24% of participants had some joint hypermobility without meeting the hEDS criteria.[ref]
Related article: Genetic variants linked to POTS
Mast Cell Activation Syndrome and hEDS:
Similar to the overlap between POTS and hEDS, there is an unusually high number of people diagnosed with hEDS along with mast cell activation syndrome (MCAS). Some people have all three diagnoses – POTS, hEDS, and MCAS.
A recent study showed the overlap between MCAS, POTS, and hEDS diagnosis in medical records was 31%.[ref]
Is it totally clear that there is a link between MCAS, POTS, and hEDS?
Well… no. hEDS and MCAS have newly evolving clinical criteria. POTS is often self-diagnosed without a clinical test. There is a lot of murkiness here in the research.
A recent review article in the journal Clinical Reviews of Allergy and Immunology sums it up: “All three clinical entities are controversial in either existence or pathogenesis. MCAS is a poorly defined clinical entity, and many studies do not adhere to the proposed criteria when establishing the diagnosis. Patients previously diagnosed with EDS hypermobility type may not meet the new, stricter criteria for hEDS but may be for a less severe hypermobility spectrum disorder (HSD). The pathophysiology of POTS is still unclear. An evidence-based, common pathophysiologic mechanism between any of the two, much less all three conditions, has yet to be described.”[ref]
A biological basis for the connection between MCAS, POTS, and hEDS:
Mast cells are part of the immune system, reacting as part of the body’s first line of defense against invaders. When a mast cell is activated, such as in response to an allergen or viral particle, it will degranulate and release mediators such as histamine, heparin, tryptase, and/or serotonin. Additionally, mast cells can release cytokines such as TNF-alpha or IL-4 in response to specific activators.
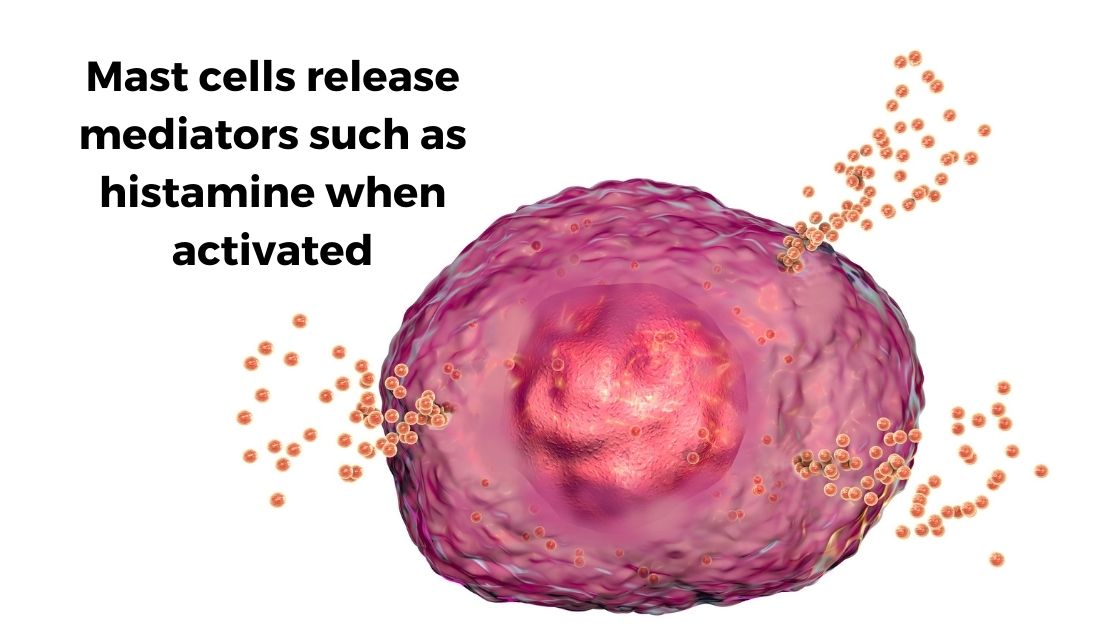
Mast cells are also important in wound healing and repair, and mast cells can reside in connective tissue. Mast cells are found abundantly in the skin, gastrointestinal tract, and respiratory tract. Additionally, mast cells can modulate heart rate by releasing histamine or tryptase.[ref]
There are several overlaps between mast cells and hypermobility Ehlers-Danlos syndrome:
- In the joints, mast cells are an essential part of the pathology of osteoarthritis. Tryptase released from mast cells induces inflammation and causes cartilage breakdown.[ref]
- In skin scarring, mast cells may also play an important role. Many studies show a higher number of mast cells or mast cell activation in scars.[ref]
- In tendon injuries, mast cells play an important role in modulating inflammation, pain, and healing. Mast cells are found not only in tendon injuries but also near peripheral nerves and may be involved in pain communication.[ref]
- Higher tryptase levels were found in a study of 35 families with skin and connective tissue abnormalities.[ref]
Related article: Read more about mast cell activation syndrome and genetics.
Fibromyalgia or ME/CFS and Ehlers-Danlos Syndrome:
Some patients with Ehlers-Danlos syndrome are misdiagnosed as having either fibromyalgia or ME/CFS. In patients (without genetic mutations in the collagen genes) who are diagnosed with hEDS or hypermobility spectrum disorder, fibromyalgia or chronic fatigue may be coexisting conditions.[ref]
A study involving 63 fibromyalgia or ME/CFS diagnosed patients found 81% met the criteria for hypermobility syndrome and 18% met the criteria for hEDS.[ref]
Clinicians reported in the journal Clinical and Experimental Rheumatology that most of their hEDS patients also met the criteria for CFS.[ref]
Ehlers-Danlos Genotype Report
Lifehacks: Research on Natural Solutions for EDS
Before I explain the research studies on possible solutions to help with Ehlers-Danlos symptoms, I want to share a good resource for anyone needing help with navigating EDS: Connective Tissue Coalition. From the site: “Connective Tissue Coalition (CTC) empowers individuals and families affected by Ehlers-Danlos Syndrome (EDS), Marfan Syndrome, and Loeys-Dietz Syndrome (LDS) through advocacy, research, and support.” Importantly, there is information on how to navigate the Social Security system in the US for getting disability.
Talk with your doctor, of course, before implementing any changes.
Physical Interventions for EDS
Related Articles and Topics:
Histamine Intolerance
Chronic headaches, sinus drainage, itchy hives, problems staying asleep, and heartburn — all of these symptoms can be caused by the body not breaking down histamine very well. Your genetic variants could be causing you to be more sensitive to foods high in histamine. Check your genetic data to see if this could be at the root of your symptoms.
TNF-Alpha: Higher innate levels of this inflammatory cytokine
Do you feel like you are always dealing with inflammation? Joint pain, food sensitivity, etc.? Perhaps you are genetically geared towards a higher inflammatory response. Tumor necrosis factor (TNF) is an inflammatory cytokine that acts as a signaling molecule in our immune system.
Rheumatoid Arthritis Genes
Rheumatoid arthritis is caused by an immune system attack on the joints, causing thickening and inflammation of the joint capsule. It is caused by a combination of genetic susceptibility and environmental triggers.