Key takeaways:
~ Neuropilins (NRPs) are cell receptors that have several roles, including in neuronal development and promoting the growth of blood and lymph vessels.
~ Genetic variants in the neuropilin (NRP1, NRP2) genes are linked to autism, migraines, lymphedema, and heart disease.
~ NRP1 and NRP2 are receptors for SARS-CoV-2.
~ NRPs are located in the endothelium lining blood vessels, on neurons in the brain, and in certain immune system cells — all key locations linked to neurological and long Covid symptoms experienced after exposure to the SARS-CoV-2 spike protein.
In this article, I’ll cover background information on these receptors, along with genetic variants that impact their function.
Members will see their genotype report below and the solutions in the Lifehacks section. Consider joining today.Neuropilin 1 and Neuropilin 2: From brain development to blood vessels
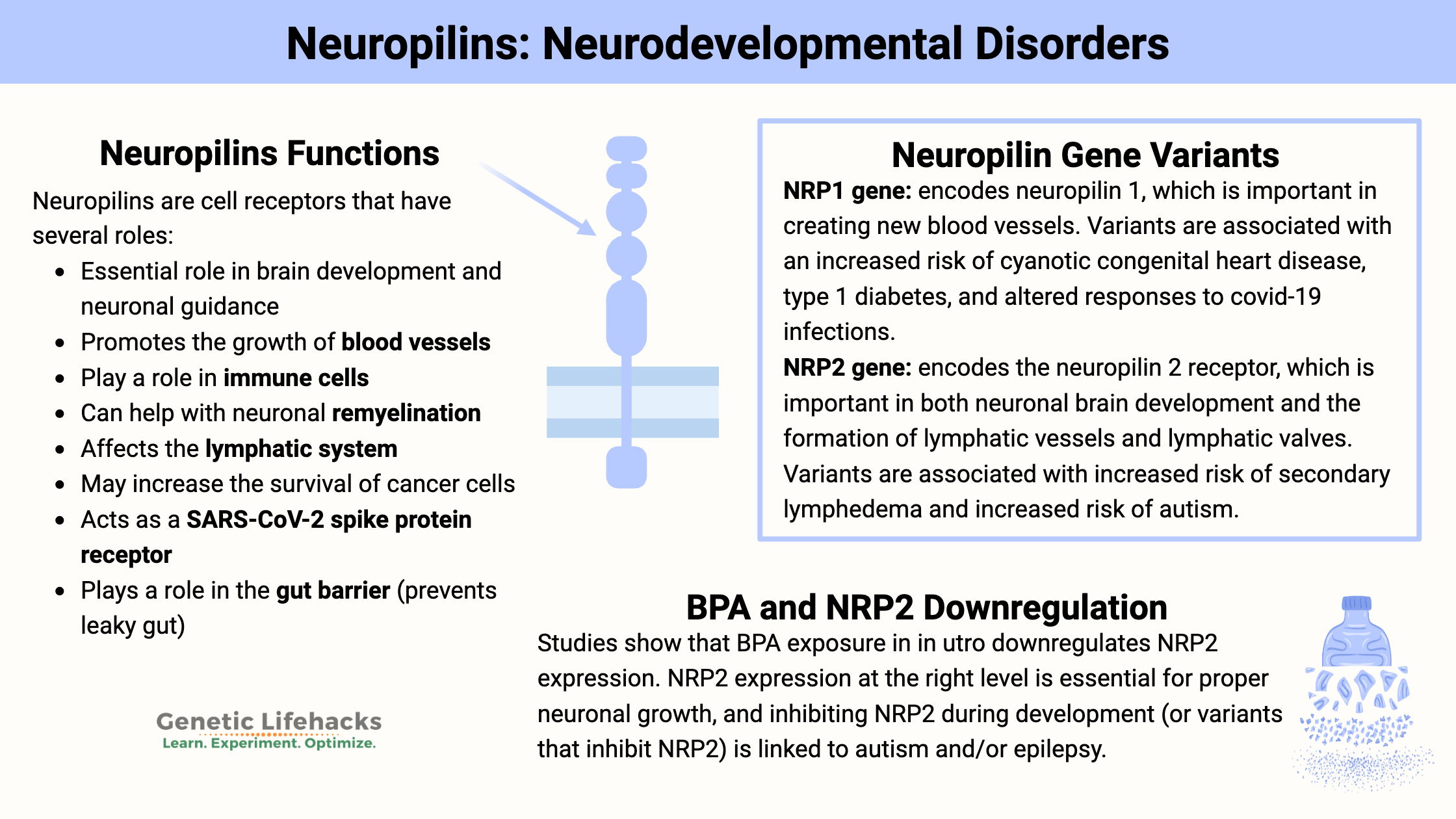
Neuropilins are glycoproteins, which means structurally, they contain a protein combined with a carbohydrate group. They were originally identified for their role in guiding axons to the right place during brain development. Since then, researchers have discovered many roles for neuropilins. Neuropilins act as receptors and are found on the surface of cells, including on neurons and epithelial cells. [ref]
Neuropilin Functions:
- Essential role in brain development and neuronal guidance
- Promotes the growth of blood vessels
- Play a role in immune cells
- Can help with neuronal remyelination
- Affects the lymphatic system
- May increase the survival of cancer cells
- Acts as a SARS-CoV-2 spike protein receptor
- Plays a role in the gut barrier (prevents leaky gut)[ref]
Blood vessels and lymphatic vessels:
In addition to their role in the nervous system and brain development, NRP1 (neuropilin 1) and NRP2 (neuropilin 2) are the main regulators of blood and lymphatic vessel growth. They can also bind the hepatocyte growth factor.[ref] Neuropilin 1 and 2 both act as a co-receptor for VEGF (vascular endothelial growth factor) as well as other growth factors.
For example:
- Neuropilin 1 (NRP1) promotes the growth of blood vessels
- Neuropilin 2 (NRP2) promotes the growth of lymph vessels.
Neuronal growth:
Additionally, neuropilins 1 and 2 act as co-receptors with semaphorins, which are involved in the development and growth of neurons and other tissues. Semaphorins also interact with the neuropilin receptors in the immune system.[ref]
Fetal development:
Animal studies show neuropilin 1 (NRP1) is essential for fetal development, and without the NRP1 gene, mice die in utero. On the other hand, too much NRP1 is also lethal, with excessive capillaries, hemorrhaging, and nervous system defects. Neuropilin 2 (NRP2) is not essential for life, but without NRP2, mice have impaired brain formation and smaller lymphatic vessels.[ref]
Diving into Neuropilins, Neuronal Development, and Immune System Interactions:
Let’s look at some of these roles in more detail. Of note – I’m just skimming the surface here and not drawing conclusions. If you are interested in the topic of autism, I would suggest looking further into neuropilins and semaphores.
- Role in autism and brain development
- Interaction with immune response
- Mast cells and neuropilin
- Viral receptor interaction with spike protein (Covid)
- Gut barrier function and leaky gut
- Well-known role in blood vessels and lymphatic vessels
Role in neuropilin 2 in autism and possibly epilepsy:
Multiple genetic studies identified NRP2 polymorphisms as a susceptibility gene for autism.[ref][ref] (To be clear: Many genes have been identified as likely increasing susceptibility to autism.)
A new animal study explains why NRP2 could be key in some types of autism. The study showed that Neuropilin-2 and its interactions with the secreted semaphorin 3F are essential during embryonic development of the cortex region of the brain. A lack of NRP2 alters the density of certain types of neurons and reduces the inhibitory to excitatory balance in the hippocampus.[ref]
Prior studies show that deletion of semaphorin 3F in animals also causes autism-like behaviors. In animals, the deletion of Sema 3F in interneurons but not excitatory neurons during early development decreased GABAergic markers and increased epilepsy and autistic behaviors. Studies also show that deficient semaphorin 3F signaling may lead to neuroinflammation and oxidative stress.[ref]
A recent animal study looked at the expression of NRP2 in the brain when the animals were exposed to low levels of BPA in utero. The results showed that NRP2 and semaphorin 5A were downregulated in the developing brain, and male offspring exhibited autism-like behaviors.[ref]
MicroRNAs regulate the amount of protein a cell makes from a gene. MicroRNA-188 (miR-188) controls the gene expression of NRP2.[ref]
Related article: MicroRNAs
Role of neuropilin in gut barrier function (preventing leaky gut):
In addition to the well-known role in regulating blood vessels, neuropilin has recently been discovered to have an important role in how the gut barrier functions.
The epithelial cells lining the intestines play a vital role in keeping the bacteria and viruses solidly in the gut microbiome and preventing them from moving into the bloodstream. There are several different proteins (claudins, zonulin) that keep the junctions between intestinal epithelial cells tight. Neuropilin receptors on the gut epithelial cells act as a feedback loop in regulating the gut barrier function through the interaction of gut microbes with TLR2 (toll-like receptor 2, an immune system receptor for the identification of pathogens).[ref]
Essentially, more NRP1 = tight junctions.
Related article: Gut mucosal barrier
Neuropilins in the immune system:
In immune system cells, neuropilin 1 and 2 are found in regulatory T cells (T regs), macrophages, thymocytes, and dendritic cells. ref]
New research also points to neuropilin 1 as a receptor for complement split products (CSPs). The complement system is part of the immune system, acting to increase the ability of other immune cells to clear out pathogens and damaged cells. CSP fragments of C4 and C3 were found to bind with NRP1.[ref]
NRP1 –> increased complement activation
Researchers have also found that lipopolysaccharide activates toll-like receptors on macrophages in a way that attenuates NRP1 expression while significantly upregulating NRP2. The NRP2 upregulation in macrophages helps to modulate the immune response through a negative feedback loop that keeps acute inflammation from getting out of control. Depletion of NRP2 exacerbates inflammation, allowing prolonged inflammation and tissue damage.[ref]
NRP2 –> puts the brakes on, allowing for the quicker resolution of inflammation.
Mast Cells and neuropilins:
Mast cells are immune system cells best known for giving off a lot of histamines when exposed to an allergen. They are immune system sentinels found in the skin, respiratory tract, gastrointestinal tract, and heart. When encountering pathogens, allergens, cytokines, or other chemical triggers, mast cells degranulate and release inflammatory molecules, including histamine and tryptase.
Related article: Mast cell activation syndrome
Mast cells express the NRP1 and NRP2 receptors, along with the co-receptors for VEGF (vascular endothelial growth factors).[ref] Mast cells can also release VEGF when stimulated. A recent study found that people with mastocytosis, a disease that causes excessive mast cell activation, have higher levels of VEGF when symptomatic. It correlates to higher levels of vascular permeability and endothelial dysfunction.[ref]
Neuropilin 1: SARS-CoV-2 receptor
Neuropilin 1 (NRP1) has been identified in a bunch of studies as being a receptor for SARS-CoV-2. While much focus was on ACE2, which was identified early on as the entry mechanism for both SARS and SARS-CoV-2, it turns out that several other cell receptors (including NRP1) can be a co-receptor that binds to the spike protein.[ref][ref][ref]
While the spike protein can bind to NRPs, the ACE2 receptor binding is about twice as efficient. Thus, most research has focused on ACE2. However, the NRP receptor binding may be important in the neurological symptoms and loss of smell in people with SARS-CoV-2 infections.[ref]
When doctors examine post-mortem brain tissue, they have found the presence of some of the SARS-CoV-2 viral particles in brain capillaries and neurons. One possible entry route is NRP1. Analyses show that NRP1 is upregulated in COVID-19 patients. The ACE2 receptor is not usually found in neurons, so researchers think that the spike protein binding to NRP1 may be important in neurological symptoms after Covid.[ref]
NRP1 and Epstein-Barr virus, NRP2 and cytomegalovirus
In addition to SARS-CoV-2, the neuropilins are also involved in the way that other viruses can get into cells. For example, NRP1 is a receptor for Epstein-Barr virus (EBV), Human T-cell Lymphotropic virus type 1, and enterovirus 71. NRP2 is a receptor for cytomegalovirus.[ref]
The important connection here – neuropilins are also found in the intestinal barrier epithelial cells and could be an entry point for viruses in the intestines.
Neuropilin 1 in vascular permeability:
The endothelium is the single layer of cells lining the blood vessels. It is more than just a lining, though, the endothelium also regulates how molecules, such as nutrients and oxygen, come in and out of the bloodstream. It is a ‘selective barrier’ that can adapt to different tissues and different localized needs.
Vascular permeability is the term applied to how open the cellular junctions are in the endothelium, allowing plasma and other things to flow in and out of the blood vessels. In certain diseases, vascular hyperpermeability can cause edema and swelling.
Neuropilin 1 (NRP1) is found throughout the endothelium. In addition to its role in blood vessel formation, recent research shows that NRP1 is also important in promoting vascular permeability. Currently, researchers are looking at targeting NRP1 with drugs in diseases such as diabetic retinopathy to prevent edema in the eye.[ref]
Lymphedema:
Rare mutations in the NRP1 and NRP2 genes are linked to significantly increased susceptibility to lymphedema, which is swelling due to the accumulation of lymph fluid in the legs or arms.
While VEGF (vascular endothelial growth factor) is best known for its role in promoting the growth of blood vessels, two subtypes of VEGF are also important in the development of the lymphatic system. Neuropilin interacts with VEGF in the promotion of the growth of vessels (blood and lymph).
More specifically, NRP1 is involved in the lymphatic valves, and NRP2 is important in lymphatic vessel growth.[ref] Changes to the lymphatic system can result in lymphedema due to the lymph fluid leaking out of the lymph vessels instead of circulating normally.
Related article: Lymphedema
NRP1 in menstrual migraines:
A large genome-wide association study looked for genetic variants related to menstrual migraines. A surprising finding was that a variant in NRP1 (neuropilin 1) was the strongest link to menstrual migraines. It differed from other migraine genome-wide studies, adding to the evidence that the vascular system is involved in migraines for some people. Neuropilin 1 is also involved in the vascular repair of the endometrium in the menstrual cycle.[ref]
Related article: Migraine genes
Cancer growth and neuropilins:
Tumors grow quickly and need blood vessels to supply them with more nutrients. Thus, reducing the growth of blood vessels into a tumor is one way to cut off growth. Currently, there is a lot of research into how targeting neuropilins can help to decrease blood vessel growth in cancer. Additionally, neuropilins play a role in how cells migrate in several types of tissues. Thus, targeting neuropilins may help to decrease metastasis in cancers.[ref]
Semaphorins and NRP1/2:
I mentioned above that semaphorins are another type of signaling molecule that can bind to neuropilin 1 and 2 — usually alongside another semaphorin receptor.
Semaphorins are important in fetal development of the nervous system. Beyond their role in early development, semaphorins are also important in nerve function and the immune system.
Semaphorins in the immune system bind with the NRP1 and NRP2 receptors on macrophages and neutrophils. When semaphorins bind to neutrophils, they inhibit the migration of neutrophils. With macrophages, certain semaphorins promote macrophages towards a role in the resolution of inflammation, while other semaphorins promote macrophages to be pro-inflammatory.[ref]
Autoimmune diseases:
Neuropilin 1 is a key characteristic of a type of T helper cell that is self-reactive. Most T helper cells keep the immune system in check, but self-reactive T helper cells have been identified as a key in systemic autoimmune diseases. By identifying neuropilin 1 as a key differentiator of the self-reactive T helper cells, researchers may be able to target the cells.[ref]
A recent animal study on autoimmune diabetes (type 1) showed that the loss of NRP1 on intraislet cells critically impacts Treg cells in the pancreas.[ref] Genetic variants in NRP1 were recently identified in a large type 1 diabetes study.[ref]
Neuropilin Genotype Report
Lifehacks:
Keep in mind that neuropilin 1 and 2 are important in how blood vessels are formed. While blocking blood vessel formation may be positive in specific tissues in cancer patients, in general, significantly blocking the neuropilins could negatively impact wound healing, ovulation, menstruation, blood pressure, and possibly immune function.[ref]
Related Articles and Topics:
Nitric Oxide Synthase (NOS3): Heart Health, Blood Pressure, and Healthy Aging
References:
Battin, Claire, et al. “Neuropilin-1 Acts as a Receptor for Complement Split Products.” Frontiers in Immunology, vol. 10, 2019, p. 2209. PubMed, https://doi.org/10.3389/fimmu.2019.02209.
Chapoval, Svetlana P., and Achsah D. Keegan. “Perspectives and Potential Approaches for Targeting Neuropilin 1 in SARS-CoV-2 Infection.” Molecular Medicine, vol. 27, no. 1, Dec. 2021, p. 162. BioMed Central, https://doi.org/10.1186/s10020-021-00423-y.
Charfeddine, Salma, et al. “Long COVID 19 Syndrome: Is It Related to Microcirculation and Endothelial Dysfunction? Insights From TUN-EndCOV Study.” Frontiers in Cardiovascular Medicine, vol. 8, 2021, p. 745758. PubMed, https://doi.org/10.3389/fcvm.2021.745758.
Fan, Sai-Hou, et al. “Functional Polymorphisms of the Neuropilin 1 Gene Are Associated with the Risk of Tetralogy of Fallot in a Chinese Han Population.” Gene, vol. 653, May 2018, pp. 72–79. PubMed, https://doi.org/10.1016/j.gene.2018.02.027.
Gudowska-Sawczuk, Monika, and Barbara Mroczko. “The Role of Neuropilin-1 (NRP-1) in SARS-CoV-2 Infection: Review.” Journal of Clinical Medicine, vol. 10, no. 13, June 2021, p. 2772. PubMed, https://doi.org/10.3390/jcm10132772.
Hosseinpour, Marziyeh, et al. “Neuropilin-2 Rs849563 Gene Variations and Susceptibility to Autism in Iranian Population: A Case-Control Study.” Metabolic Brain Disease, vol. 32, no. 5, Oct. 2017, pp. 1471–74. PubMed, https://doi.org/10.1007/s11011-017-0024-2.
Kyrou, Ioannis, et al. “Not Only ACE2—the Quest for Additional Host Cell Mediators of SARS-CoV-2 Infection: Neuropilin-1 (NRP1) as a Novel SARS-CoV-2 Host Cell Entry Mediator Implicated in COVID-19.” Signal Transduction and Targeted Therapy, vol. 6, no. 1, Jan. 2021, pp. 1–3. www.nature.com, https://doi.org/10.1038/s41392-020-00460-9.
Marone, Gianni, et al. “Mast Cells and Basophils in Inflammatory and Tumor Angiogenesis and Lymphangiogenesis.” European Journal of Pharmacology, vol. 778, May 2016, pp. 146–51. PubMed, https://doi.org/10.1016/j.ejphar.2015.03.088.
Patterson, Bruce K., et al. “Persistence of SARS CoV-2 S1 Protein in CD16+ Monocytes in Post-Acute Sequelae of COVID-19 (PASC) up to 15 Months Post-Infection.” Frontiers in Immunology, vol. 12, 2022. Frontiers, https://www.frontiersin.org/articles/10.3389/fimmu.2021.746021.
Raveney, Ben JE, et al. “Neuropilin‐1 ( NRP1 ) Expression Distinguishes Self‐reactive Helper T Cells in Systemic Autoimmune Disease.” EMBO Molecular Medicine, Sept. 2022. DOI.org (Crossref), https://doi.org/10.15252/emmm.202215864.
Roy, Sohini, et al. “Multifaceted Role of Neuropilins in the Immune System: Potential Targets for Immunotherapy.” Frontiers in Immunology, vol. 8, 2017. Frontiers, https://www.frontiersin.org/articles/10.3389/fimmu.2017.01228.
Stuart, Lynda M. “In Gratitude for MRNA Vaccines.” New England Journal of Medicine, edited by Elizabeth G. Phimister, vol. 385, no. 15, Oct. 2021, pp. 1436–38. DOI.org (Crossref), https://doi.org/10.1056/NEJMcibr2111445.
Sulpice, Eric, et al. “Neuropilin-1 and Neuropilin-2 Act as Coreceptors, Potentiating Proangiogenic Activity.” Blood, vol. 111, no. 4, Feb. 2008, pp. 2036–45. PubMed, https://doi.org/10.1182/blood-2007-04-084269.
Vilella, F., et al. “Single-Cell RNA Sequencing of SARS-CoV-2 Cell Entry Factors in the Preconceptional Human Endometrium.” Human Reproduction (Oxford, England), vol. 36, no. 10, Sept. 2021, pp. 2709–19. PubMed, https://doi.org/10.1093/humrep/deab183.
Wu, Suping, et al. “Association of the Neuropilin-2 (NRP2) Gene Polymorphisms with Autism in Chinese Han Population.” American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics, vol. 144B, no. 4, June 2007, pp. 492–95. PubMed, https://doi.org/10.1002/ajmg.b.30495.
Xu, Jing, et al. “Genetic Variants at 10p11 Confer Risk of Tetralogy of Fallot in Chinese of Nanjing.” PloS One, vol. 9, no. 3, 2014, p. e89636. PubMed, https://doi.org/10.1371/journal.pone.0089636.