Key takeaways:
~ When looking at the root causes of migraines, three pathways seem to be involved: vascular, neural, and pain/nerve pathways.
~ Genetic variants in these different pathways may help you to pinpoint your triggers of migraines.
~ There are natural options backed by research that may help with preventing migraines – or at least decreasing the number of migraine days. Genetics may give you the starting point to figure out which prevention strategies are more likely to work for you.
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Consider joining today.
What is a migraine and why does it happen?
Migraines impact over a billion people globally, and women are three times as likely than males to suffer from them.[ref] That’s a lot of people who have experienced the feeling of mental fogginess, pain, sensitivity to light, stiff neck, and an overwhelming desire to find a dark, quiet place to hide from the world.
Migraine symptoms generally include:
- headache (usually lasting 4 to 72 hours)
- sensitivity to light, sounds, and smells
- nausea or vomiting
- sensory disturbances, aura (sometimes)
Somehow that list of symptoms doesn’t really do migraines justice.
For many, migraines are more than a headache — and, usually, the pain isn’t even the worst part. Instead, it’s the altered ability to think, irritability, nausea, slowed reflexes, and body temperature fluctuations. Or maybe that is just me?
The different types of migraines:
- migraines with aura (about 1/3 of migraineurs get auras)
- migraines without aura
- hemiplegic migraines (rare numbness/tingling on one side of the body)
Some people get premonitory or prodromal symptoms, which may appear up to a day or two before the migraine. Irritability, fatigue, food cravings, stiff neck, sensitivity to sounds, and yawning are some of the warning signs. On a PET scan, these symptoms accompany an increased blood flow to the hypothalamus.[ref]
Chronic migraines affect around 2% of the population. If you have more than 8 migraine days each month, your migraines are considered chronic.[ref]
What is going on in your brain when you have a migraine?
In the 1960s, researchers found that people with migraines had higher levels of a serotonin metabolite, 5-hydroxyindoleacetic acid (5-HIAA), in their urine. This has prompted a lot of research over the past five decades into the relationship between the serotonergic system and migraines.
More recently, researchers have also investigated how CGRP (calcitonin gene-related peptide), neuroinflammation, and altered ion levels impact migraines.[ref]
Brain electrical storm: Migraines are often described as an excitatory state in the brain – also known as a state of hypersynchrony. Other researchers describe migraines as the brain over-responding to stimulus.[ref]
Research provides a lot of competing theories, but no simple answers…
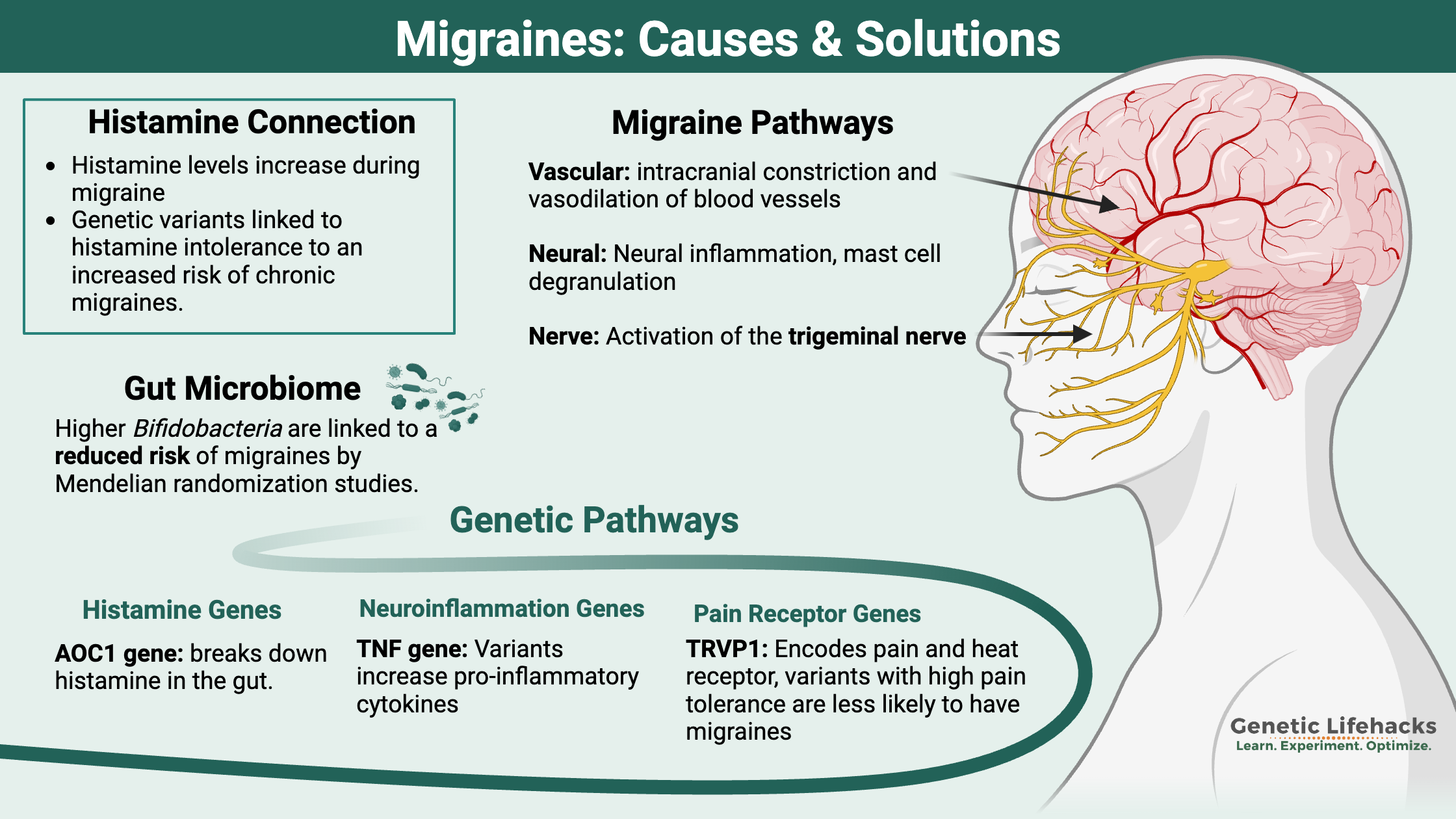
Migraine research breaks down into three paths:
- vascular causes (intracranial constriction and vasodilation of blood vessels)
- neural events (hyperexcitability and cortical spreading depression)
- nociceptive causes (pain pathways, activation of the trigeminal nerve, neuropeptides)
Vasodilation and migraines:
Part of what happens in your brain during a migraine suggests vasodilation, which is the widening of blood vessels in your brain. Vasodilation causes decreased blood pressure. The vasodilation reaction results from an initial period of vasoconstriction. This may be happening only in the brain and not in the rest of the body. The blood vessels inside the cranium don’t always act in the same manner as the rest of the body.[ref]
These vascular (blood vessel) causes may be due (at least in part) to serotonin signaling.
Serotonin (5-hydroxytryptamine, 5-HTP) is a neurotransmitter that causes the signal to flow from one neuron to the next. It is found in abundance in both the digestive system and the brain.
Brain morphology, the way the neurons in the brain are shaped and formed, is also affected by serotonin.
Serotonin is just the signal and it needs a receptor to bind to and cause an action. There are different serotonin receptors found throughout the brain and intestinal tract, causing diverse effects of serotonin.[ref]
One big link between migraines and serotonin is that triptans, the most commonly used migraine prescription medication, work by amplifying the serotonin signal. Triptans act on the serotonin receptors (5-HT1B) in the blood vessels of the brain, constricting them and inhibiting the release of neuropeptides.[ref]
Genetic studies show a link between migraine susceptibility and a type of genetic variant known as a variable number tandem repeat in a serotonin transporter gene.[ref]
Using PET scans on people who had been migraine-free for at least 48 hours, researchers have begun studying the 5-HT1B (serotonin) receptor in the brain. These scans showed migraine patients had lower 5-HT1B binding than those without migraines. The results of the study suggest two possibilities:[ref]
- Low numbers of serotonin receptors may either be causal (low serotonin causing migraines);
- or, that serotonin receptors decrease over time because of repeated exposure to migraines.
In other words, it isn’t determined if low serotonin causes migraines or if migraines cause changes to the expression of serotonin receptors.
(Check your serotonin variants in the genotype report below)
Melatonin and migraines:
Researchers also found low melatonin levels in people with chronic migraines when compared to a control population. It is notable, especially because serotonin is needed for the reaction in the pineal gland that produces melatonin.[ref]
tryptophan -> serotonin -> melatonin
One study explains that serotonin levels are low between migraine attacks, but are shown to increase at the beginning of a migraine. This initial surge of serotonin causes vasoconstriction and is thought to be part of the aura phase. When serotonin then breaks down (which happens pretty quickly), serotonin levels drop, causing vasodilation and headache pain.[ref]
Related article: Serotonin: How your genes affect this neurotransmitter
CGRP in migraines:
Another key player in migraines is CGRP. CGRP stands for calcitonin gene-related peptide and is released from the trigeminovascular system. The trigeminovascular system includes both the trigeminal nerve neurons and the cerebral blood vessels.
The trigeminal nerve is the largest cranial nerve. It branches to the eyes and the jaw. Here’s a graphic showing where the trigeminal nerve is located:
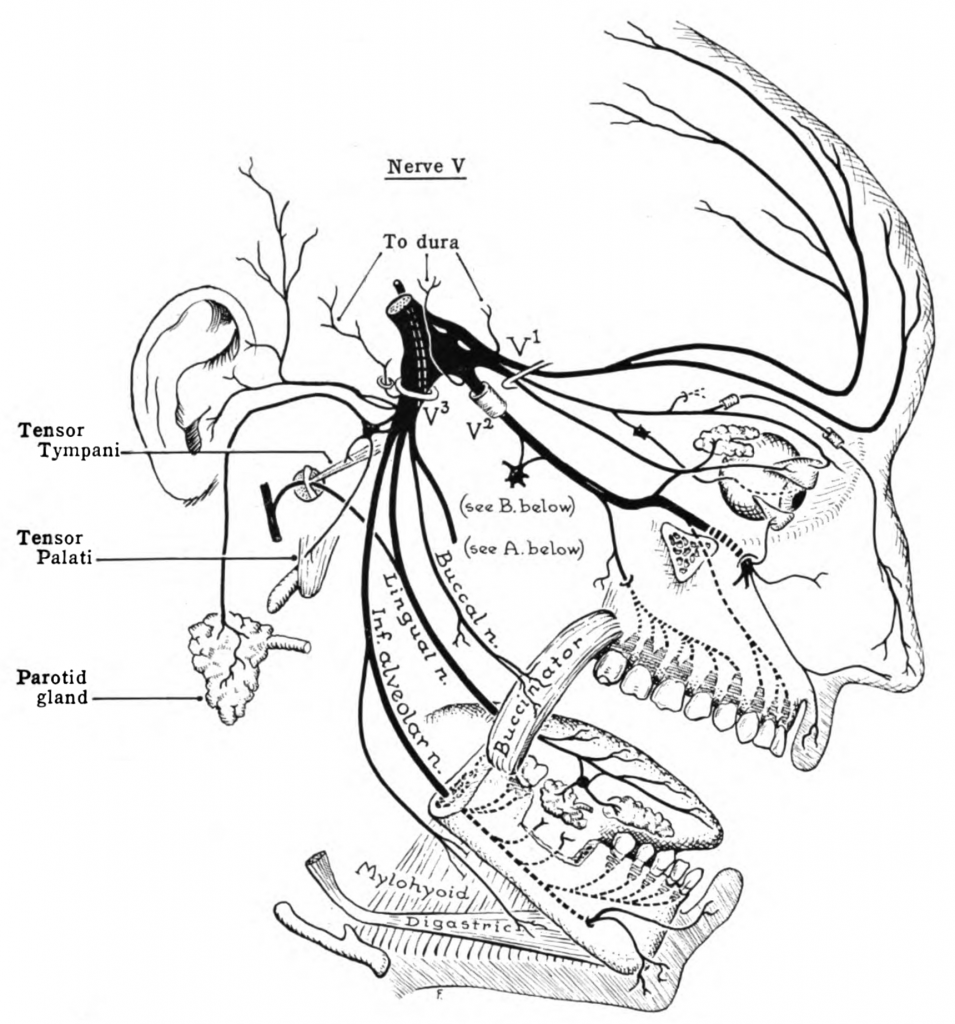
For me, the trigeminal nerve explains the moving pain points of a migraine – from the temple to the eyebrow and then to causing my teeth to ache.
The sensory nerve fibers of the trigeminal nerve can activate the release of neuropeptides, including CGRP, substance P, and neurokinin A. CGRP is a potent vasodilator making it important for both migraines and normal blood pressure regulation in the body. It is also thought to influence the pain portion of migraines.[ref][ref]
There are a lot of new migraine drugs being developed that focus on CGRP. However, a CGRP isn’t solely responsible for migraines, and medications that target CGRP only work for a portion of patients.[ref]
CGRP also can bind to the amylin 1 receptor in the brain. New research points to this being a possible pathway involved in the way that is involved in causing migraines.[ref]
(Check your CGRP variants in the genotype report below)
Neuroinflammation in migraines:
The release of CGRP seems to activate receptors on several different cell types, including mast cells.
The activated mast cells can degranulate, releasing histamine and pro-inflammatory compounds. This degranulation of mast cells causes a “prolonged state of excitation in meningeal nociceptors”. The meninges line the skull, and nociceptors are pain receptors.[ref][ref]
Here is an image of the meninges, which includes three layers surrounding the brain (dura mater, arachnoid, and pia mater).
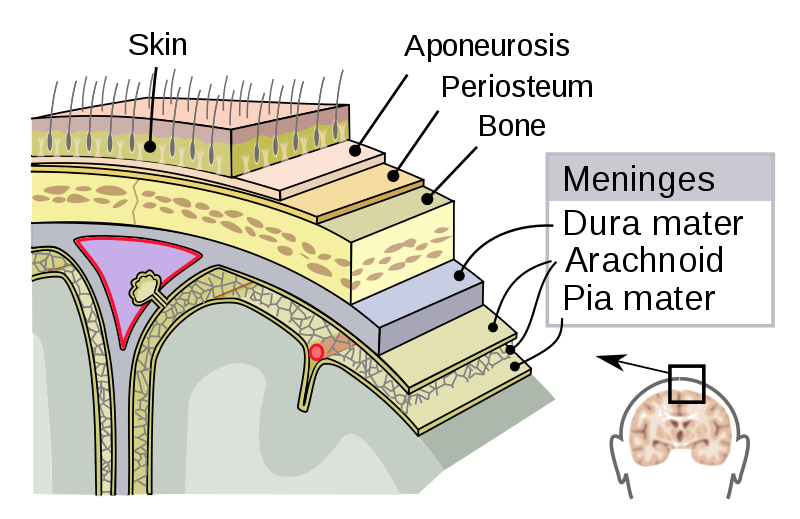
The brain itself doesn’t have pain receptors, but there are pain receptors and blood vessels throughout the meninges.
Mast cells are part of the body’s immune system and are found in all of our body tissues. They stand ready to release a payload of histamine, serotonin, tryptase, and inflammatory cytokines when activated by a pathogen, allergic reaction, or other signaling molecules (such as CGRP).[ref]
(Check your histamine variants in the migraine genotype report below)
Some researchers theorize that mast cells and neuroinflammation are at the root of migraine pathology. Here are two reasons pointing to mast cell degranulation as a root cause:
- Histamine, released when mast cells degranulate, is elevated during migraine attacks.[ref]
- Tryptase is also released during degranulation and is thought to sensitize pain receptors.[ref]
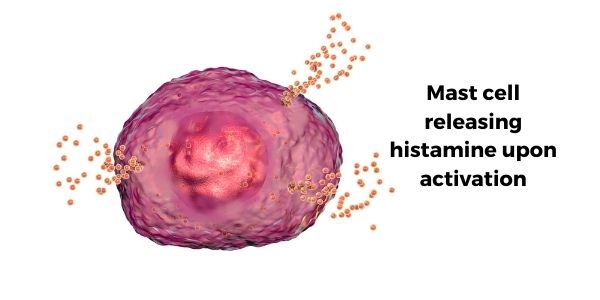
Other researchers theorize that central sensitization is at the root of migraines. Central sensitization involves enhanced signaling through pain pathways and is caused by overexcited pain receptors and decreased inhibition. This term applies to various pain-related conditions such as peripheral neuropathy, IBS, and migraines.[ref]
Related article: Mast cells: MCAS, genetics, and solutions
Ion levels: Can an electrolyte imbalance cause migraines?
Another observation of researchers is that ion levels are often altered in migraineurs.
Sodium, potassium, calcium, and chloride ions are integral parts of how neurons fire. Neurons send signals to other neurons by transmitting electrical impulses carried by positive and negative ions.
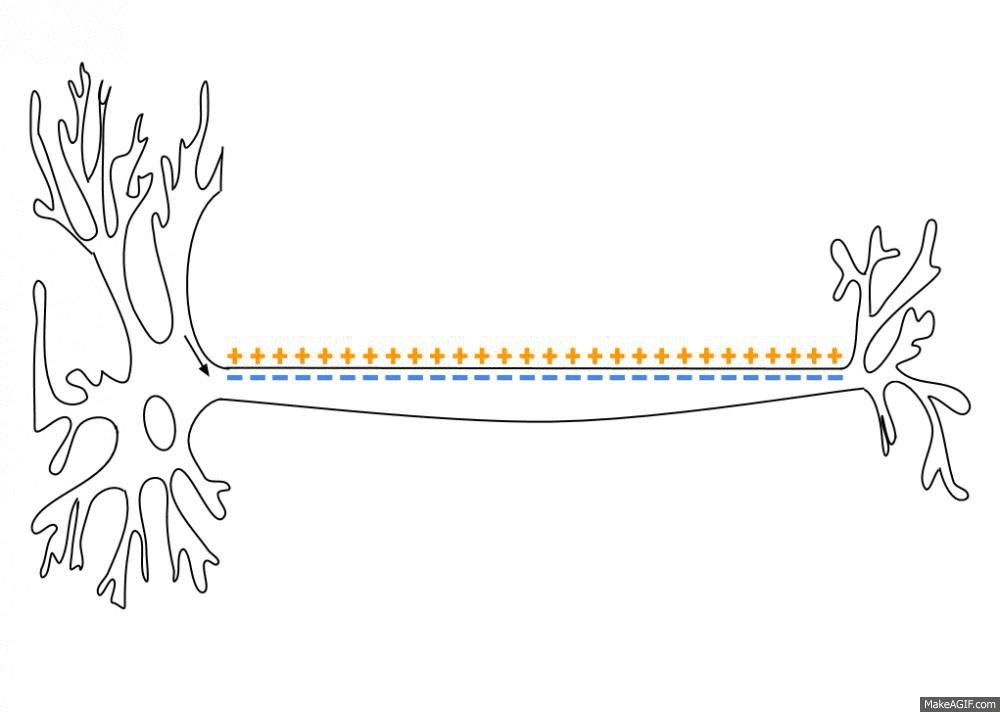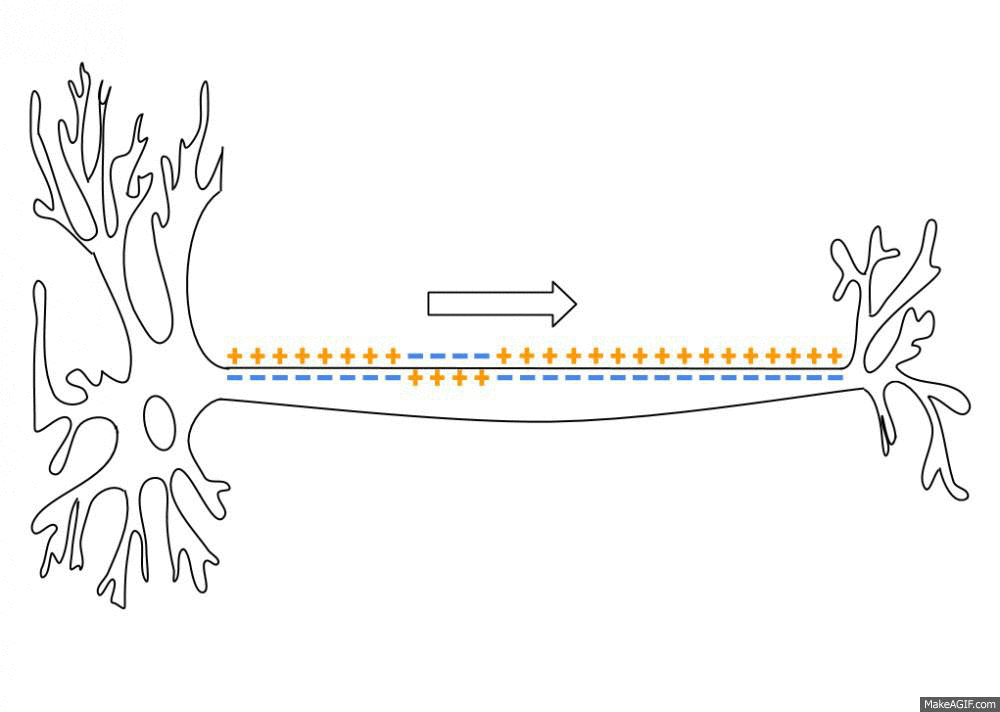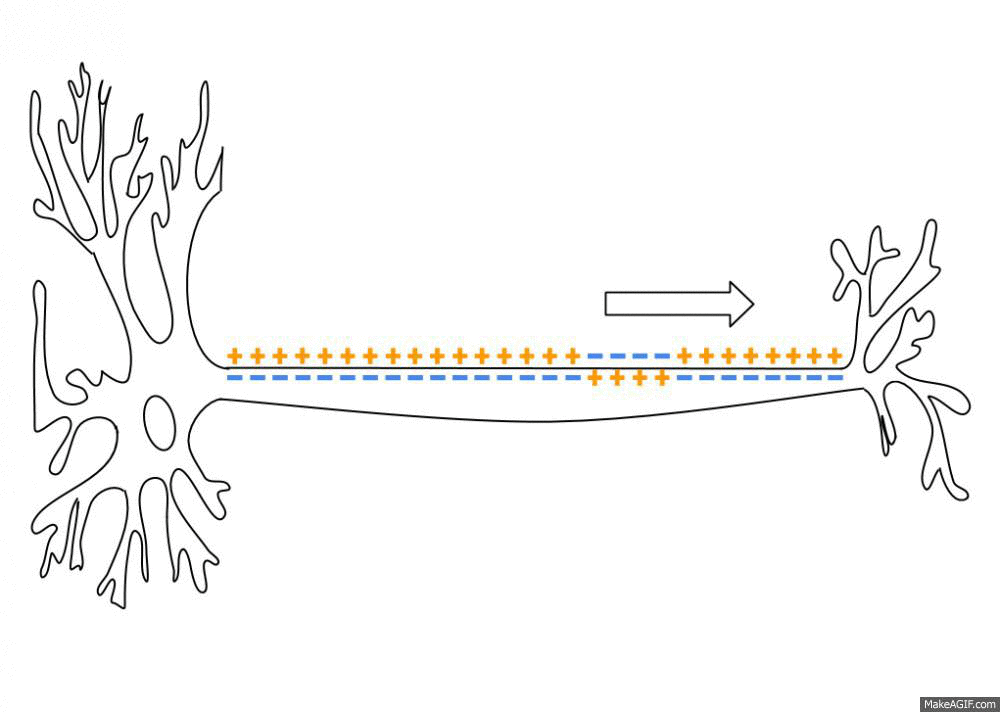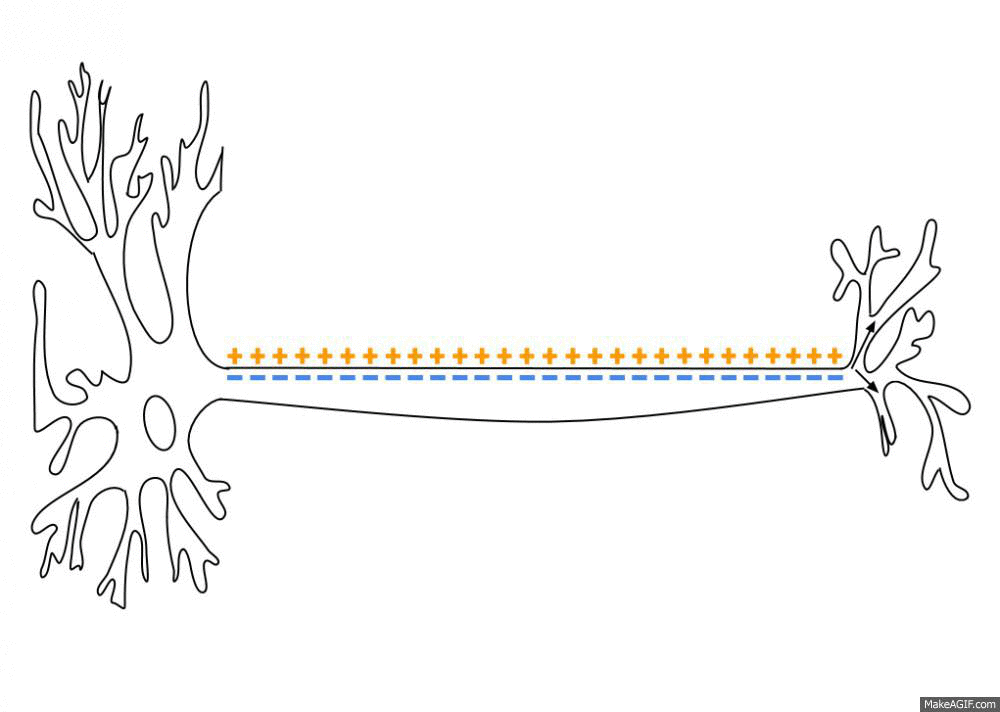
A recent study found that sodium (Na) levels were higher in the cerebrospinal fluid (CSF) of people with migraines compared with a control group. It backs up previous work that also showed an increase in CSF sodium levels during a migraine. The other ion levels – calcium, potassium, and magnesium – did not change, nor did the sodium levels in the plasma.[ref][ref]
There have been large studies trying to determine if sodium intake affects migraine risk. One study found that for women with low BMI and men at any BMI, a low sodium intake slightly increased the risk of headaches and migraines.[ref] Another study found that people who have migraines have, on average, lower magnesium levels than a healthy control group.[ref]
Mechanoreceptors in migraines:
So far we’ve gone over vasodilation, neuroinflammation, and ion channels that drive neurons to fire, which are all loosely based on the levels of different mediators (serotonin, histamine, CGRP, etc.).
Adding to the complexity of the system here is that there are mechanoreceptors that can be activated due to the shear force on the sides of the blood vessels due to changes in blood flow. Essentially, your blood vessels can detect changes in the force of the blood flow by activating mechanoreceptors in the endothelium. One review paper explains: “This interplay of chemical and mechanical forces can initiate a relentless vicious circle of neuronal sensitization and sterile inflammation, which supports the persistence of migraine pain”[ref]
Most of your body is ok with some swelling, such as swelling from a sprained ankle or swelling from a splinter in your finger. The brain is very sensitive to swelling — your skull is a fixed size, and there isn’t room for much change in the volume.
In addition to detecting changes in shear flow in blood vessels, mechanoreceptors in the brain are very sensitive to volume changes. For example, reduced glymphatic outflow can cause a slight swelling in the brain. CGRP release and/or the activation of mast cells causes neuroinflammation, which is also detected by mechanoreceptors.
TRP channels and Piezo receptors are mechanosensitive receptors found in the central nervous system and likely involved in migraines.
TRP channels (e.g. TRPV1, TRPM8) and Piezo1/2 are ion channels on neurons that are activated in response to temperature, chemicals, and mechanical force. The channels allow ions to flow and then transmit a signal from one neuron to the next.[ref]
TRPV1 receptors play a significant role in migraine-related mechanical pain. Found in high amounts in the arteries of chronic migraine sufferers, these receptors increase arterial sensitivity to pain. Animal studies using show that TRPV1 receptor activation leads to sensitization of the trigeminal pain system. However, TRPV1 antagonist drugs haven’t worked all that well for migraines in human clinical trials.[ref] So there is more going on than just TRP activation.
(Check your TRP variants in the genotype report below)
Gut microbiome and migraines:
Many studies have found differences in the gut microbiome of migraine patients compared to healthy, age- and sex-matched controls. However, the question is whether the changes are due to diet or another coincidental factor — or if the gut microbiome changes are playing a role in causing migraines.[ref]
Mendelian randomization is a statistical method used to determine causality based on known genetic phenotypes. A recent Mendelian randomization study looked at the question of whether the gut microbiome changes were causative for migraines. The researchers used known genetic variants that are linked to having higher or lower levels of certain gut bacteria. They found that certain gut bacteria are causally related to the increased risk of migraines. Interestingly, higher Bifidobacteria are linked to a reduced risk of migraines.[ref]
What causes the aura in migraines?
A migraine aura is the altered sensory perception, such as visual alterations like sparkles and the waviness of images.
The Mayo Clinic explains that “A visual aura is like an electrical or chemical wave that moves across the visual cortex of your brain. The visual cortex is part of your brain that processes visual signals. As the wave spreads, you might have visual hallucinations.” Sensory auras may also cause tingling and problems with speaking.
The aura is thought to result from cortical spreading depression (CSD). CSD is a massive depolarization of neurons that occurs in the brain. A wave of hyperexcitability of the neurons is followed by a wave of inhibition. This depolarization causes the release of potassium, hydrogen, nitric oxide, and glutamate ions, as well as arachidonic acid.[ref]
Here is a visual:
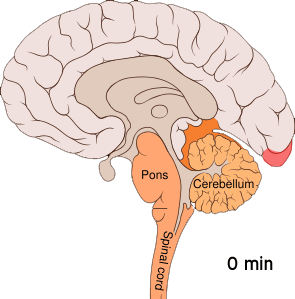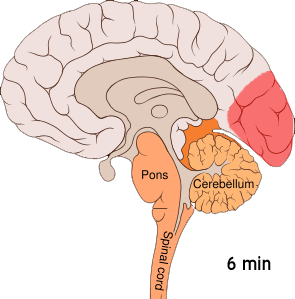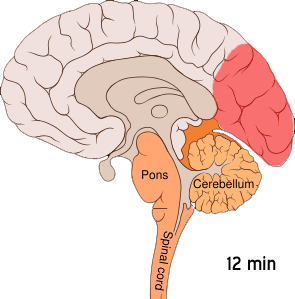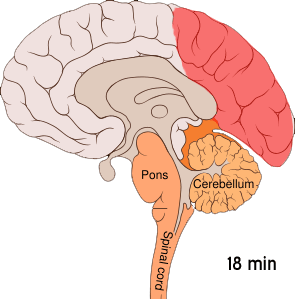
This cortical-spreading depression causes the pain receptors in the meninges to start firing after a bit of a delay. The firing of the pain receptors continues for a long while. Animal studies show that inducing CSD by various means caused the pain receptors to start firing about 14 minutes later.[ref]
Bringing the various research studies together:
Sometimes when reading a bunch of research studies on a topic, I feel like the analogy of the blind men describing different parts of an elephant.
To recap, here’s what research shows on migraines:
- Involvement of the serotonin system can cause changes in blood vessel dilation.
- The trigeminal neurovascular system releases CGRP, which is a vasodilator that also acts on mast cells.
- Activation of mast cells causes sterile neuroinflammation. Mast cells release histamine, tryptase, and other inflammatory cytokines.
- The inflammation acts on the meninges, the lining of the cranium, causing pain.
- Mechanoreceptors are activated by changes in blood vessels and sterile inflammation in the brain.
- If the migraine is preceded by an aura, the cortical spreading depression may be what is triggering the nociceptors (pain receptors) in the meninges to fire.
Migraine triggers:
People often identify various triggers for their migraines. Traveling, foods, stress, hormones, odors, sleep deprivation, and flashing/bright lights often top the list.
One problem with identifying migraine triggers is that the premonitory or prodromal symptoms (up to a day or two ahead of the migraine) may overlap with what people identify as triggers.
- Food cravings are one prodromal symptom, and various food triggers are often blamed. Thus, it is hard to separate the food craving due to prodromal symptoms from the unusual food that may seem like a trigger.
- Altered sleep patterns are another thing identified as a trigger which could be a prodromal symptom. Instead of altered sleep causing a migraine, the prodromal migraine causes altered sleep.
- Bright light sensitivity could also fall into this conundrum.
While sometimes difficult to pin down, it does seem that there are specific triggers for some people. These include certain foods, scents, hormonal changes, and weather changes.
Which foods trigger migraines?
A lot of people have identified specific foods as being likely to cause migraines for them. Some of these include gluten, wine, cheese, and chocolate.
Genetics may come into play here:
- Some people are likely to be intolerant to suddenly consume a lot of foods with high tyramine levels. (Read more about tyramines increasing blood pressure)
- Genetic variants also link histamine intolerance to an increased risk of chronic migraines. (Read more about genes related to histamine intolerance.)
- Other top triggers for migraines include MSG and beer.[ref][ref]
Studies show, though, that ‘overall dietary patterns’ don’t relate to migraine risk. Dietary patterns refer to the big picture of your diet – vegetarian, carnivore, keto, fruitarian, etc. One study did show that women with migraines eat a slightly higher amount of fat and a slightly lower amount of protein on average.[ref][ref]
Why do scents trigger migraines?
Some people are sensitive to odors and find that certain smells (floral, perfumes, strong cleaning chemicals, paint, gasoline, plastics) can trigger migraines.[ref]
It isn’t just all in your head (pun intended). A small study looked at cerebral blood flow in people with migraines vs. a control group. The migraineurs had altered blood flow in certain areas of the brain when stimulated with odors.[ref]
What causes menstrual migraines?
One really common migraine trigger for many women is the change in estrogen levels around the time of their period or during ovulation. Migraines often go away for these women during pregnancy and after menopause.
Researchers have found that it is the drop in estrogen that usually triggers migraines. Giving women estrogen or estrogen mimics before a period and then discontinuing at the same time that estrogen naturally drops will trigger a migraine for many women.
Estrogen acts by binding to an estrogen receptor and activating it. Estrogen receptors are located on the cell surface of some cell types and are most abundant in the cell nucleus, where the act to modify the translation of a bunch of different genes related to growth. In the brain, estrogen receptors are present in neurons, including some of the neuron types implicated in migraines such as those producing CGRP.[ref]
Mast cells have estrogen and progesterone receptors. The fluctuation of estrogen can cause mast cell degeneration and subsequent migraine pain.
Genetics and Migraines:
For more than three decades, researchers have dug into how and why genetic differences impact migraines. Understanding the genes involved can help to point to what is really going wrong in a migraine.
Familial hemiplegic migraines are a rare type of migraine caused by a genetic mutation. This affects around 1 in 10,000 people. However, understanding the genetic causes here adds to understanding the pathology of migraines.
The genes that cause familial hemiplegic migraines include CACNA1A, ATP1A2, SCN1A, and PRRT2. These genes are involved in the way ions move between neurons in the brain. CACNA1A is a calcium channel that moves calcium ions in the terminals of neurons, playing an important role in modulating the release of neurotransmitters. Other genes related to familial hemiplegic migraines relate to sodium or potassium channels in neurons.[ref]
For people with normal migraines, there are more than a hundred common genetic variants that add or subtract from the risk. All together, these common variants come together to increase or decrease an individuals susceptibility to having migraines.
Mendelian randomization studies use vast amounts of data to tease out whether a known genetic impact is causal for a condition – or just something associated with the condition. For migraines, Mendelian randomization studies show that the genetic variants that increase the risk of migraines are likely not causing increased stroke, heart attacks, or Alzheimer’s. There is also no likely causal association of the genetic variants that cause increased coffee consumption or smoking playing any role in migraines.
However, Mendelian randomization studies do show a causal link in genes related to migraines and sleep disorders, such as insomnia. Similarly, people who have genetically predicted higher serum calcium levels are also more likely to have migraines. And people who genetically are likely to have higher vitamin D levels are at a decreased statistical risk of migraines.[ref]
Migraine Genotype Report:
Researchers estimate that migraines are ~50% due to heredity factors (such as genetic variants), which combine with environmental factors (hormones, high histamine foods) to cause migraines.[ref][ref]
How can you use this info? Identifying the genetic variants you carry that influence migraine susceptibility may help you figure out why YOU get migraines, and, hopefully, which migraine ‘lifehacks’ will be most effective for you. Keep in mind that your 23andMe or AncestryDNA only covers part of your genes, so this section doesn’t cover every variant linked to migraines.
Pain Receptors Genes Associated with Migraines:
TRPM8 gene: encodes the cold and menthol receptors. When skin temperatures decrease by 15 degrees C, it hurts due to the activation of this pain receptor.[ref]
Check your genetic data for rs10166942 (23andMe v4, v5; AncestryDNA):
- C/C: decreased risk of migraines[ref][ref]
- C/T: slightly decreased risk of migraines
- T/T: higher risk of migraines, less sensitive to cold[ref]
Members: Your genotype for rs10166942 is —.
TRPV1 gene: encodes a receptor sensitive to temperature and capsaicin (spicy foods)
Check your genetic data for rs8065080 (23andMe v5; AncestryDNA):
- T/T: typical receptor function; more likely to have chronic migraines[ref]
- C/T: typical receptor function; more likely to have chronic migraines
- C/C: higher pain tolerance to cold, heat[ref]; less TRPV1 receptor activation[ref] less likely to have chronic migraines[ref]
Members: Your genotype for rs8065080 is —.
CGRP Pathway Genes Associated with Migraines:
CGRP and brain-derived neurotrophic factor (BDNF) coexpress on the trigeminal nerve terminals.[ref]
BDNF Gene: codes for brain-derived neurotrophic factor
Check your genetic data for rs6265 (23andMe v4, v5; AncestryDNA):
- T/T: increased risk of migraines[ref]
- C/T: slightly increased risk of migraines
- C/C: typical risk of migraines
Members: Your genotype for rs6265 is —.
MMP16 gene: Large genome-wide association studies found a genetic variant on chromosome 8 near the MMP16 gene that is consistently associated with a reduced risk of migraines (without aura). The MMP16 gene codes for an enzyme involved in the breakdown of the extracellular matrix and tissue remodeling.
Check your genetic data for rs10504861 (23andMe v4, v5; AncestryDNA):
Members: Your genotype for rs10504861 is —.
Methylation Pathway Genes Associated with Migraines:
Methylation is important in converting serotonin into melatonin.
NNMT gene: The nicotinamide-N-methyltransferase (NNMT) gene codes for an enzyme that transfers a methyl group from SAMe to nicotinamide. There are links between the variants of this gene and increases in homocysteine levels.
Check your genetic data for rs694539 (23andMe v4, v5; AncestryDNA):
- TT: a 4-fold increase in the risk of migraines in women[ref]
- CT: typical migraine risk
- CC: decreased migraine risk
Members: Your genotype for rs694539 is —.
MTHFR gene: Several studies have linked the MTHFR C677T variant to an increased risk of migraines, but not all of the studies agree. This one may be a mild increase in risk or perhaps for just certain population groups.[ref]
Check your genetic data for rs1801133 (23andMe v4, v5; AncestryDNA):
- G/G: typical risk of migraine
- A/G: slightly increased risk of migraines
- A/A: slightly increased risk of migraine[ref][ref][ref]
Members: Your genotype for rs1801133 is —.
Serotonin and Vascular Pathways:
C7orf10 gene: codes for an enzyme involved in the production of glutarate from tryptophan (precursor for serotonin) and lysine.
Check your genetic data for rs4379368 (23andMe v4, v5; AncestryDNA):
- C/C: typical risk of migraines
- C/T: decreased risk of migraines
- T/T: decreased risk of migraines (Caucasian ancestry)[ref] (opposite found in a Chinese population)[ref]
Members: Your genotype for rs4379368 is —.
SLC6A4 gene: codes for a serotonin transporter.
Check your genetic data for rs2066713 (23andMe v4, v5; AncestryDNA):
- G/G: typical risk of migraines
- A/G: decreased risk of migraines
- A/A: decreased risk of migraines[ref]
Members: Your genotype for rs2066713 is —.
Note: a lot of other genetic variants in the serotonin transport and receptor genes have been studied and found not to be involved in migraine risk.
PHACTR1 gene: involved in blood pressure, coronary artier disease, and migraine risk[ref]
Check your genetic data for rs9349379 (23andMe v4, v5; AncestryDNA):
- G/G: typical risk of migraines
- A/G: increased risk of migraines
- A/A: increased risk of migraines[ref]
Members: Your genotype for rs9349379 is —.
Histamine / Mast Cell Activation Pathway Genes Associated with Migraines:
AOC1 gene: codes for diamine oxidase, which breaks down histamine in the gut.
Check your genetic data for rs1049793 (23andMe v4; older AncestryDNA files):
- C/C: typical
- C/G: increased risk of migraines
- G/G: increased risk of migraines[ref] (reduced DAO enzyme, which breaks down histamine from foods)
Members: Your genotype for rs1049793 is —.
Check your genetic data for rs10156191 (23andMe v4; AncestryDNA):
- C/C: typical
- C/T: increased risk of migraines, especially in women
- T/T: increased risk of migraines, especially in women[ref] (reduced DAO enzyme, which breaks down histamine from foods)
Members: Your genotype for rs10156191 is —.
Inflammatory Pathway Genes Associated with Migraines:
TNF gene: tumor necrosis factor-alpha is one of the body’s main pro-inflammatory cytokines.
Check your genetic data for rs3093664 (23andMe v5; AncestryDNA):
- G/G: increased risk of menstrual migraines[ref] (increased inflammatory response)
- A/G: increased risk of menstrual migraines
- A/A: typical
Members: Your genotype for rs3093664 is —.
Check your genetic data for rs1800750 (23andMe v4, v5; AncestryDNA):
- G/G: typical risk of migraines
- A/G: increased risk of migraines
- A/A: increased risk of migraines[ref] (increased inflammatory response)
Members: Your genotype for rs1800750 is —.
Check your genetic data for rs1800629 (23andMe v4, v5; AncestryDNA):
- A/A: increased risk of migraines[ref] (increased inflammatory response)
- A/G: increased risk of migraines
- G/G: typical
Members: Your genotype for rs1800629 is —.
IL1A gene: codes for interleukin-1A, which is part of the body’s inflammatory response
Check your genetic data for rs17561 (23andMe v4; AncestryDNA):
- C/C: typical
- A/C: increased risk of migraines
- A/A: increased risk of migraines[ref] (increased inflammatory response)
Members: Your genotype for rs17561 is —.
Ion and neurotransmitter pathways associated with migraines:
KCNK18 gene: encodes a potassium receptor that regulates the excitability of neurons in pain, including the trigeminal nerve
Check your genetic data for rs869025175 (23andMe v5):
- I/D: rare mutation, strongly linked to migraines due to potassium receptor loss of function mutation[ref]
- I/I: typical
Members: Your genotype for rs869025175 is —.
MTDH gene: “MTDH is known to downregulate EAAT2 (excitatory amino acid transporter 2), a major glutamate transporter, and could thus affect glutamate regulation at a synaptic level”[ref]
Check your genetic data for rs1835740 (23andMe v4, v5; AncestryDNA):
Members: Your genotype for rs1835740 is —.
Cholesterol Pathway Genes Associated with Migraines:
LRP1 gene: codes for an LDL cholesterol receptor. Migraine intensity and frequency link (in a small study) to cholesterol levels.[ref]
Check your genetic data for rs11172113 (23andMe v4, v5; AncestryDNA):
- C/C: slightly decreased risk for migraines[ref]
- C/T: slightly decreased risk for migraines
- T/T: typical risk for migraines
Members: Your genotype for rs11172113 is —.
Menstrual Migraine Specific Variants:
NRP1 gene: encode neurolipin 1
Check your genetic data for rs2506142 (AncestryDNA):
- G/G: 2-fold increased risk of menstrual migraines[ref]
- A/G: increased risk for menstrual migraines
- A/A: typical
Members: Your genotype for rs2506142 is —.
Lifehacks: Preventing Migraines
Included here are lifestyle changes, research studies on medication efficacy, and natural supplements with clinical trials for migraine relief.
Good sleep hygiene for migraine prevention:
Circadian Rhythm and Migraines:
A study of over 2000 people with migraines found that early morning onset was quite frequent, found in about 40% of migraineurs. The study also found that people with chronic migraines were more tired after circadian disruption, such as staying up late, and less able to cope with being active during times when they weren’t normally active.[ref] All in all, this points to the importance of sticking to a regular sleep schedule for people prone to migraines.
The early morning onset could be due to increased histamine levels in the early morning hours (causing mast cell degranulation) or due to increased TNF-alpha at night.[ref][ref]
Blocking blue light at night increases melatonin production. Try turning down overhead lights and shutting off electronics a few hours before bed. While it seems like such a mild and simple change, the impact of light at night on circadian rhythm is huge. Give it a try for a week and see how much eliminating electronics and bright light at night can do for your sleep quality and migraine frequency.
Meditation for Migraines:
A study of mindfulness meditation did not find that it statistically reduced migraines, although there was a trend toward fewer migraines per month.[ref]
Why does exercise help migraines?
Exercise and Migraines:
While exercise is a known trigger for some people for migraines, it also raises BDNF levels. A study of exercise and migraines suggests that regular activity may help decrease the frequency of migraines in the long run.[ref]
Yoga:
A clinical trial of yoga found that it did reduce the frequency and intensity of migraines.[ref]
Which medications work to treat migraines?
Talk with your doctor if you have questions about specific medications. This information on medication research is educational and not treatment advice.
Migraine drug classifications include triptans, ergotamines, CGRP monoclonal antibodies, and botox.
One thing to note about clinical trials on preventative medications is that most consider ‘effectiveness’ to be a 50% reduction in migraine frequency. If you normally have six days of migraines per month, a medication is considered effective if it reduces migraines to 3 days/month.
Thus, the goal of these medications seems to be a reduction in frequency rather than actually eliminating or curing migraines.
Ergotamines:
Ergotamines were originally derived from ergot, a fungus that infects rye and other grains.
Ergot is famous for outbreaks in the Middle Ages, causing what was known as St. Anthony’s fire. Ingesting grains infected with the fungus caused extreme vasoconstriction, causing a severe burning sensation in the limbs. Ergots also affect neurotransmission and cause hallucinations.
Ergotamines are available in prescription form for migraine attacks. They bind to serotonin receptors, causing vasoconstriction as well as other effects.[ref] Migergot is one brand name that combines ergot with caffeine. There are possible serious side effects (black box warning).
Triptans:
Triptans are often the first line of prescription medication offered to people with migraines, and they are used as a prophylactic to prevent migraines.
Triptans target specific serotonin receptors in the brain (5HT1B, 5HT1D). These receptors are located in the trigeminal nerve endings, so it is thought that activating them prevents the release of CGPG and substance P.[ref]
How well do triptans work? It depends… For some people, they seem to work a little better than aspirin or ibuprofen.
A meta-analysis of 133 randomized controlled trials looked at the efficacy of triptan medications for migraines. The conclusion was “most triptans are associated with equal or better outcomes compared with NSAIDs, ASA (aspirin), and acetaminophen”. Looking at the data, triptans relieved pain in two hours for between 42 and 76% of patients. NSAIDs, aspirin (ASA), and acetaminophen relieved pain in two hours for 46 – 52% of patients.[ref]
What the meta-analysis showing that triptans and aspirin/NSAIDs are about equivalent doesn’t tell us is whether those ~50% of people that triptans work for are the same people that aspirin/NSAIDs work for…
In other words, if aspirin/NSAIDs don’t work for you, would triptans be a better option? Or are 50% of people just out of luck when it comes to effective migraine medications?
Another study compared naproxen sodium with a combo of naproxen sodium along with a triptan (prescription med). The results showed that only naproxen sodium was statistically significant in reducing migraines, and triptan was not. 43% of subjects who just took naproxen sodium for migraine had a 50% reduction in migraine frequency (compared to 17% with the expensive prescription med).[ref] The current cost of triptan is well over $200 for nine tablets[source], while naproxen sodium is about $4 at Target.
CGRP receptor inhibitors in clinical trials:
Several small-molecule GCRP inhibitors have been tested and are in current clinical trials for migraine usage. They are generally termed ‘gepants’. A new monoclonal antibody targeting CGRP, fremanezumab, was tested in a group of over 700 study participants. The injection of fremanezumab once a month reduced migraine frequency from 9 per month to 5 per month. It was compared with a placebo which reduced migraine frequency from 9 per month to 6.5 per month.[ref] The CGRP drugs are being heavily invested in and investigated. At some point, there will likely be studies showing genetic connections to who they work best for.
Botox:
OnabotulinumtoxinA, called Botox, is a neurotoxin injected into the face and approved to prevent chronic migraines (and wrinkles).
The PREEMPT study on the initial phase III clinical trial showed a statistical decrease in migraine days, going from 8.4 to 6.6 migraine days per month on average.[ref]
One study compared botox injections to amitriptyline, a tricyclic antidepressant used for migraines. The results showed that both were about equally effective, with around 70% of trial participants having a reduction in the number of days of migraines each month.[ref]
Natural supplements for migraine relief:
Riboflavin:
Riboflavin, or vitamin B2, works to prevent migraines for some people. A placebo-controlled study investigated riboflavin for migraine prevention. The trial used 400 mg of riboflavin daily for 3 months. About 60% of the participants had reduced migraine frequency using riboflavin (compared to the 15% placebo effect).[ref]
Riboflavin is also effective for lowering homocysteine and is important in the methylation cycle. So this may be a good ‘hack’ to try if you have either of the methylation cycle pathway variants listed above.
Magnesium to prevent migraines:
The studies showing that people with migraines have lower magnesium levels combine well with a trial of magnesium to prevent migraines. If you think you are low in magnesium, it may be a good supplement to try.[ref][ref] Foods high in magnesium include legumes, nuts, chocolate, and avocados.
Decreasing TNF-Alpha:
If your genetic risk factors for migraines include the TNF-alpha variants, you may want to try decreasing TNF-alpha through some natural supplements.
☑ Black seed oil – Nigella sativa – has been shown to reduce TNF-alpha. In an animal study, it reduced mast cell degranulation in the meninges.[ref][ref] You can get black seed oil at your local health food store or online.
☑ Curcumin has been shown to reduce inflammatory cytokines in the brain, including TNF-alpha. A clinical trial found that the combo of curcumin and CoQ10 effectively reduced the number of migraines.[ref][ref] Another clinical trial found that nano-curcumin (80mg/day for two months) reduced the number and frequency of migraines.[ref]
Melatonin supplements:
A couple of clinical trials have evaluated the efficacy of melatonin in preventing migraines. One study compared 3 mg of melatonin to placebo and amitriptyline (25 mg). Melatonin showed to be significantly better than placebo, but about the same as amitriptyline. Another clinical trial compared melatonin to placebo and sodium valproate (prescription migraine med). Melatonin again seems superior to placebo and similar to sodium valproate in reducing migraines. Additionally, it also seems to have fewer side effects than sodium valproate.[ref][ref]
Tryptophan also is the precursor amino acid for melatonin, so supplementing with tryptophan (with carbs, without other branch-chain amino acids) has been shown in studies to increase melatonin levels.
Blocking blue light at night (from TV, laptop, phone, bright overhead lights) also naturally increases your melatonin levels.
CoQ10:
A placebo-controlled clinical trial showed that CoQ10 statistically reduced the frequency and severity of migraines. Study participants took 400 mg/day of CoQ 10 for three months.[ref] Another study found that the combination of CoQ10, riboflavin, and magnesium reduced the number of migraine days from 6 per month to 4 per month. It also reduced the severity of migraines.[ref]
Aspirin:
Studies show that taking an aspirin daily works to reduce migraine frequency, for some people. Of course, you would need to weigh the slight risk of increased bleeding with daily aspirin; talk with your doctor if you have questions on this.
- A study that looked back over medical records for a decade found that people taking aspirin regularly to prevent migraines had fewer migraines.[ref]
- A meta-analysis of 8 different migraine prevention studies showed that aspirin statistically reduced migraine frequency at a dose of 325mg/day (a single regular-strength aspirin).[ref]
Tryptophan to increase serotonin and melatonin:
Serotonin is derived from the amino acid tryptophan. A study using a tryptophan-depletion diet showed that it worsened headaches and nausea in migraine sufferers.[ref]
Tryptophan is generally abundant in diets that contain enough protein. However, if you don’t eat a lot of protein, you may want to track the amount of tryptophan you eat and increase your intake of tryptophan-containing foods such as salmon, eggs, poultry, cheese, and spinach.[ref]
Alternatively, tryptophan is available as a powdered supplement. For it to cross the blood-brain barrier, you must eat tryptophan with carbs and without other branch-chain amino acids.
Migraine Diet Option:
Does the ketogenic diet prevent migraines? A ketogenic diet works for certain types of epilepsy, so it makes sense that it may also help with migraines. And it might help a little, maybe, for some people… or perhaps just cleaning up your diet, in general, would help.
A study of 96 overweight women with frequent migraines investigated whether a keto diet would help. Half of the women went on a keto diet for six months, and the other half went on a standard lower-calorie diet. Both groups met with a nutritionist to plan out their diets. The women on the keto diet went from a baseline migraine frequency of 2.9 migraines per month to 2.2 migraines per month at month 6. The women on the standard diet went from 3.2 migraines/month to 2.4 migraines per month.[ref]
Folate-rich foods: A diet high in folate-rich foods is theorized to help with migraines, but the research on this is somewhat limited.[ref] If you carry the MTHFR or NNMT variants, it may be well worth tracking your folate consumption (from foods, not folic acid) for a month or so. Cronometer.com is a free app for tracking micronutrients. Read about: Folate-Rich Foods and Recipe Ideas
Topical migraine relief:
Heat, Cold, and Peppermint:
The TRPM8 cold/menthol receptor is active in the meningeal inflammation of a migraine. Studies show that cooling the head (ice pack) or peppermint oil (menthol) on the forehead is effective for migraine relief for some people.[ref]
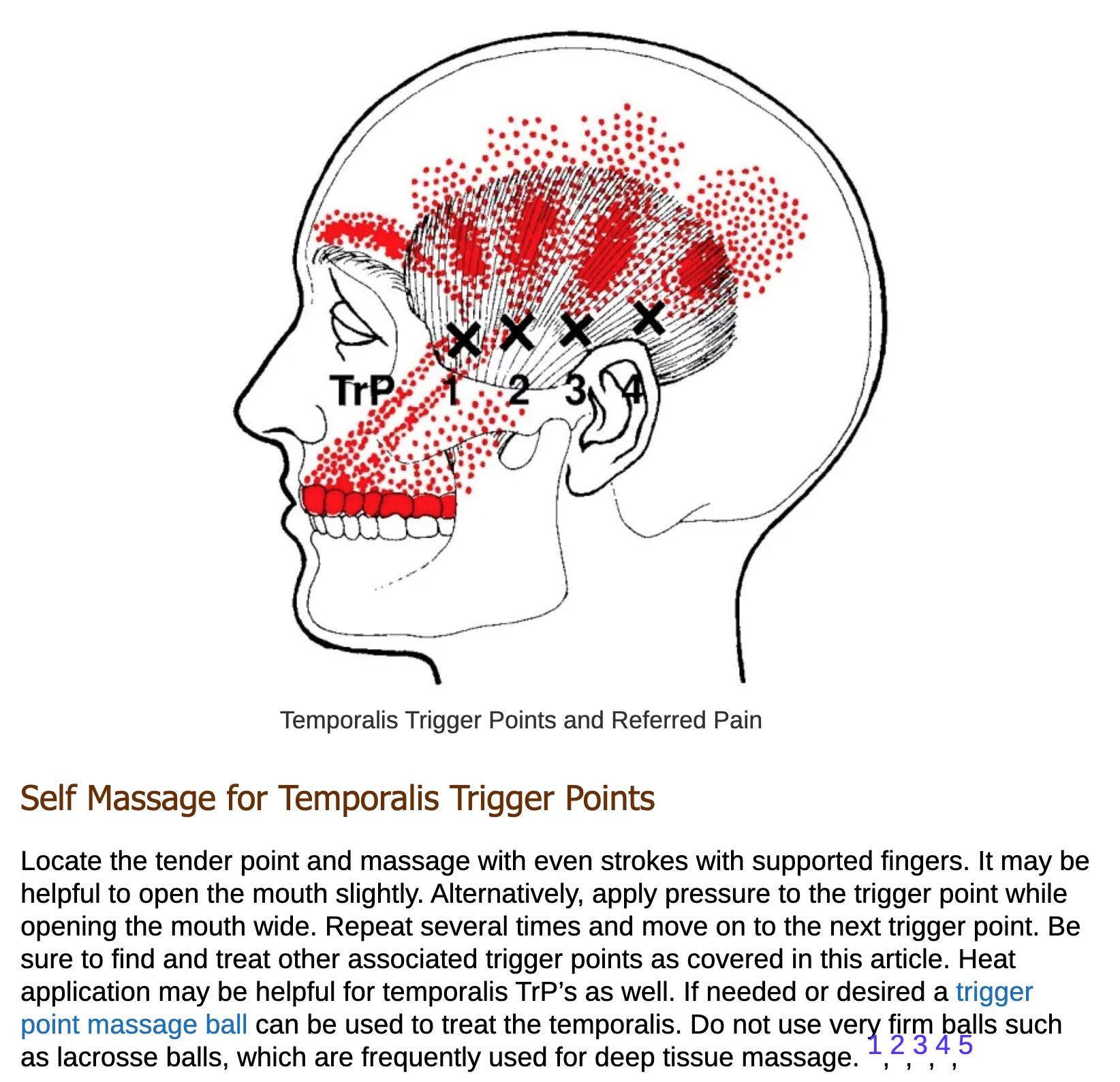
Trigger Point Therapy:
Myofascial trigger points are associated with the pain of migraines. These trigger points are often found in the areas around the trigeminal nerve.
Here is a good resource on the topic: Screenshot from Temporalis Trigger Points (try rubbing the spots marked with the X when you have a migraine).
If you like books, check out Trigger Point Therapy for Headaches and Migraines.
Electrical stimulation:
A randomized sham-controlled study looked at the effect of electrical stimulation at specific points in the ear. The points were aligned with the auricular branch of the vagus nerve at the external ear. Check out the reference article for more details.[ref]
Another option may be to look into manual massage of the vagus nerve — look for videos on auricular vagus nerve massage or see if you can find a massage practitioner who is familiar with it.
Physical therapy:
A randomized controlled trial showed that manual therapy had improvements in quality of life and pain intensity after four weeks (one session per week). The therapy was described as being conducted on the neck, upper trunk, and sacroiliac joints. [ref]
Recap of your genes:
| Gene | RS ID | Effect Allele | Your Genotype | Notes About Effect Allele |
|---|---|---|---|---|
| TRPM8 | rs10166942 | C | -- | decreased risk of migraines (temperature and menthol receptor gene) |
| BDNF | rs6265 | T | -- | Increased risk of migraines due to lower BDNF |
| MMP16 | rs10504861 | T | -- | Reduced risk of migraines |
| NNMT | rs694539 | T | -- | TT only: 4-fold increase in migraine risk (methylation cycle) |
| MTHFR | rs1801133 | A | -- | Increased risk of migraines (methylation cycle) |
| C7orf10 | rs4379368 | T | -- | Decreased risk of migraines (serotonin) |
| SLC6A4 | rs2066713 | A | -- | Decreased risk of migraines (serotonin) |
| AOC1 | rs1049793 | G | -- | Increased risk of migraines (histamines from foods) |
| AOC1 | rs10156191 | T | -- | Increased risk of migraines (histamines from foods) |
| TNF | rs3093664 | G | -- | Increased risk of migraines (inflammatory pathway) |
| TNF | rs1800750 | A | -- | Increased risk of migraines (inflammatory pathway) |
| TNF | rs1800629 | A | -- | Increased risk of migraines (inflammatory pathway) |
| IL1A | rs17561 | A | -- | Increased risk of migraines (inflammatory pathway) |
| KCNK18 | rs869025175 | D | -- | rare mutation (talk with your doctor) |
| MTDH | rs1835740 | T | -- | This variant is linked to glutamate regulation. Glutamate is an excitatory neurotransmitter. |
| LRP1 | rs11172113 | C | -- | Less likely to have migraines caused by cholesterol |
| NRP1 | rs2506142 | G | -- | 2-fold increased risk of menstrual migraine |
| PHACTR1 | rs9349379 | A | -- | Increased risk of migraines |
| TRPV1 | rs8065080 | C | -- | C/T: more likely to have chronic migraines; C/C: protective against chronic migraines |
Related Articles and Topics:
MTHFR and Migraines
The MTHFR C677T variant increases the risk of migraines. Learn how to check your genetic data and how to mitigate the risk.
PMS, Genetics, and Solutions
A lot of women know the moodiness and brain fog that comes with premenstrual syndrome (PMS). Studies estimate that PMS is up to 95% heritable – which means that it has a huge genetic component. Learn about the genes and find out which solutions may actually work for you.
Serotonin Genes
Serotonin is a neurotransmitter that is important in depression, sleep, and many other aspects of health. Learn how your genetic variants in the serotonin receptor genes impact their function.
Histamine Intolerance
High histamine levels can cause a variety of symptoms, including migraines, hives, sinus drainage, and stomach problems.
References:
“Temporalis Trigger Points and Referred Pain Patterns.” Triggerpointselfhelp.Com, 3 Mar. 2019, https://triggerpointselfhelp.com/temporalis-trigger-points-and-referred-pain-patterns/.
Amin, Faisal Mohammad, et al. “The Association between Migraine and Physical Exercise.” The Journal of Headache and Pain, vol. 19, no. 1, Sept. 2018, p. 83. PubMed Central, https://doi.org/10.1186/s10194-018-0902-y.
An, Xing-Kai, et al. “Association of MTHFR C677T Polymorphism with Susceptibility to Migraine in the Chinese Population.” Neuroscience Letters, vol. 549, Aug. 2013, pp. 78–81. PubMed, https://doi.org/10.1016/j.neulet.2013.06.028.
Andreeva, Valentina A., et al. “Macronutrient Intake in Relation to Migraine and Non-Migraine Headaches.” Nutrients, vol. 10, no. 9, Sept. 2018, p. 1309. PubMed Central, https://doi.org/10.3390/nu10091309.
Anttila, Verneri, et al. “Genome-Wide Meta-Analysis Identifies New Susceptibility Loci for Migraine.” Nature Genetics, vol. 45, no. 8, Aug. 2013, pp. 912–17. PubMed Central, https://doi.org/10.1038/ng.2676.
—. “Genome-Wide Meta-Analysis Identifies New Susceptibility Loci for Migraine.” Nature Genetics, vol. 45, no. 8, Aug. 2013, pp. 912–17. PubMed Central, https://doi.org/10.1038/ng.2676.
Baena, Cristina Pellegrino, et al. “The Effectiveness of Aspirin for Migraine Prophylaxis: A Systematic Review.” Sao Paulo Medical Journal = Revista Paulista De Medicina, vol. 135, no. 1, Feb. 2017, pp. 42–49. PubMed, https://doi.org/10.1590/1516-3180.2016.0165050916.
Baj, Tirthraj, and Rohit Seth. “Role of Curcumin in Regulation of TNF-α Mediated Brain Inflammatory Responses.” Recent Patents on Inflammation & Allergy Drug Discovery, vol. 12, no. 1, 2018, pp. 69–77. PubMed, https://doi.org/10.2174/1872213X12666180703163824.
Bayerer, Bettina, et al. “Single Nucleotide Polymorphisms of the Serotonin Transporter Gene in Migraine–an Association Study.” Headache, vol. 50, no. 2, Feb. 2010, pp. 319–22. PubMed, https://doi.org/10.1111/j.1526-4610.2009.01553.x.
Benemei, Silvia, et al. “Triptans and CGRP Blockade – Impact on the Cranial Vasculature.” The Journal of Headache and Pain, vol. 18, no. 1, Oct. 2017, p. 103. PubMed Central, https://doi.org/10.1186/s10194-017-0811-5.
—. “Triptans and CGRP Blockade – Impact on the Cranial Vasculature.” The Journal of Headache and Pain, vol. 18, no. 1, Oct. 2017, p. 103. PubMed Central, https://doi.org/10.1186/s10194-017-0811-5.
—. “Triptans and CGRP Blockade – Impact on the Cranial Vasculature.” The Journal of Headache and Pain, vol. 18, no. 1, Oct. 2017, p. 103. PubMed Central, https://doi.org/10.1186/s10194-017-0811-5.
—. “Triptans and CGRP Blockade – Impact on the Cranial Vasculature.” The Journal of Headache and Pain, vol. 18, no. 1, Oct. 2017, p. 103. PubMed Central, https://doi.org/10.1186/s10194-017-0811-5.
BORAN, H. Evren, and Hayrunnisa BOLAY. “Pathophysiology of Migraine.” Nöro Psikiyatri Arşivi, vol. 50, no. Suppl 1, Aug. 2013, pp. S1–7. PubMed Central, https://doi.org/10.4274/Npa.y7251.
Buldyrev, Ilya, et al. “Calcitonin Gene-Related Peptide Enhances Release of Native Brain-Derived Neurotrophic Factor from Trigeminal Ganglion Neurons.” Journal of Neurochemistry, vol. 99, no. 5, Dec. 2006, pp. 1338–50. PubMed, https://doi.org/10.1111/j.1471-4159.2006.04161.x.
Cady, Roger, et al. “SumaRT/Nap vs Naproxen Sodium in Treatment and Disease Modification of Migraine: A Pilot Study.” Headache, vol. 54, no. 1, Jan. 2014, pp. 67–79. PubMed, https://doi.org/10.1111/head.12211.
Cameron, Chris, et al. “Triptans in the Acute Treatment of Migraine: A Systematic Review and Network Meta-Analysis.” Headache, vol. 55 Suppl 4, Aug. 2015, pp. 221–35. PubMed, https://doi.org/10.1111/head.12601.
Chasman, Daniel I., et al. “Genome-Wide Association Study Reveals Three Susceptibility Loci for Common Migraine in the General Population.” Nature Genetics, vol. 43, no. 7, July 2011, pp. 695–98. www.nature.com, https://doi.org/10.1038/ng.856.
—. “Genome-Wide Association Study Reveals Three Susceptibility Loci for Common Migraine in the General Population.” Nature Genetics, vol. 43, no. 7, July 2011, pp. 695–98. www.nature.com, https://doi.org/10.1038/ng.856.
Chehl, Navdeep, et al. “Anti-Inflammatory Effects of the Nigella Sativa Seed Extract, Thymoquinone, in Pancreatic Cancer Cells.” HPB : The Official Journal of the International Hepato Pancreato Biliary Association, vol. 11, no. 5, Aug. 2009, pp. 373–81. PubMed Central, https://doi.org/10.1111/j.1477-2574.2009.00059.x.
Chiang, Chia-Chun, and Amaal J. Starling. “OnabotulinumtoxinA in the Treatment of Patients with Chronic Migraine: Clinical Evidence and Experience.” Therapeutic Advances in Neurological Disorders, vol. 10, no. 12, Dec. 2017, pp. 397–406. PubMed Central, https://doi.org/10.1177/1756285617731521.
—. “OnabotulinumtoxinA in the Treatment of Patients with Chronic Migraine: Clinical Evidence and Experience.” Therapeutic Advances in Neurological Disorders, vol. 10, no. 12, Dec. 2017, pp. 397–406. PubMed Central, https://doi.org/10.1177/1756285617731521.
Christ, Pia, et al. “The Circadian Clock Drives Mast Cell Functions in Allergic Reactions.” Frontiers in Immunology, vol. 9, July 2018, p. 1526. PubMed Central, https://doi.org/10.3389/fimmu.2018.01526.
Daubert, Elizabeth A., and Barry G. Condron. “Serotonin: A Regulator of Neuronal Morphology and Circuitry.” Trends in Neurosciences, vol. 33, no. 9, Sept. 2010, pp. 424–34. PubMed Central, https://doi.org/10.1016/j.tins.2010.05.005.
Deen, Marie, et al. “Low 5-HT1B Receptor Binding in the Migraine Brain: A PET Study.” Cephalalgia: An International Journal of Headache, vol. 38, no. 3, Mar. 2018, pp. 519–27. PubMed, https://doi.org/10.1177/0333102417698708.
Demarquay, G., et al. “Olfactory Hypersensitivity in Migraineurs: A H(2)(15)O-PET Study.” Cephalalgia: An International Journal of Headache, vol. 28, no. 10, Oct. 2008, pp. 1069–80. PubMed, https://doi.org/10.1111/j.1468-2982.2008.01672.x.
—. “Olfactory Hypersensitivity in Migraineurs: A H(2)(15)O-PET Study.” Cephalalgia: An International Journal of Headache, vol. 28, no. 10, Oct. 2008, pp. 1069–80. PubMed, https://doi.org/10.1111/j.1468-2982.2008.01672.x.
Di Lorenzo, C., et al. “Migraine Improvement during Short Lasting Ketogenesis: A Proof-of-Concept Study.” European Journal of Neurology, vol. 22, no. 1, Jan. 2015, pp. 170–77. PubMed, https://doi.org/10.1111/ene.12550.
Dodick, David W., et al. “Effect of Fremanezumab Compared With Placebo for Prevention of Episodic Migraine: A Randomized Clinical Trial.” JAMA, vol. 319, no. 19, May 2018, pp. 1999–2008. PubMed, https://doi.org/10.1001/jama.2018.4853.
Drummond, P. D. “Tryptophan Depletion Increases Nausea, Headache and Photophobia in Migraine Sufferers.” Cephalalgia: An International Journal of Headache, vol. 26, no. 10, Oct. 2006, pp. 1225–33. PubMed, https://doi.org/10.1111/j.1468-2982.2006.01212.x.
Durham, Paul L. “Diverse Physiological Roles of Calcitonin Gene-Related Peptide in Migraine Pathology: Modulation of Neuronal-Glial-Immune Cells to Promote Peripheral and Central Sensitization.” Current Pain and Headache Reports, vol. 20, no. 8, Aug. 2016, p. 48. PubMed Central, https://doi.org/10.1007/s11916-016-0578-4.
—. “Diverse Physiological Roles of Calcitonin Gene-Related Peptide in Migraine Pathology: Modulation of Neuronal-Glial-Immune Cells to Promote Peripheral and Central Sensitization.” Current Pain and Headache Reports, vol. 20, no. 8, Aug. 2016, p. 48. PubMed Central, https://doi.org/10.1007/s11916-016-0578-4.
—. “Diverse Physiological Roles of Calcitonin Gene-Related Peptide in Migraine Pathology: Modulation of Neuronal-Glial-Immune Cells to Promote Peripheral and Central Sensitization.” Current Pain and Headache Reports, vol. 20, no. 8, Aug. 2016, p. 48. PubMed Central, https://doi.org/10.1007/s11916-016-0578-4.
Ebrahimi-Monfared, Mohsen, et al. “Use of Melatonin versus Valproic Acid in Prophylaxis of Migraine Patients: A Double-Blind Randomized Clinical Trial.” Restorative Neurology and Neuroscience, vol. 35, no. 4, 2017, pp. 385–93. PubMed, https://doi.org/10.3233/RNN-160704.
Evans, E. Whitney, et al. “Dietary Intake Patterns and Diet Quality in a Nationally Representative Sample of Women with and without Severe Headache or Migraine.” Headache, vol. 55, no. 4, Apr. 2015, pp. 550–61. PubMed, https://doi.org/10.1111/head.12527.
Foods Highest in Tryptophan. https://nutritiondata.self.com/foods-000079000000000000000.html. Accessed 3 Sept. 2021.
García-Martín, Elena, et al. “Diamine Oxidase Rs10156191 and Rs2052129 Variants Are Associated with the Risk for Migraine.” Headache, vol. 55, no. 2, Feb. 2015, pp. 276–86. PubMed, https://doi.org/10.1111/head.12493.
Gasparini, Claudia Francesca, et al. “Genetic and Biochemical Changes of the Serotonergic System in Migraine Pathobiology.” The Journal of Headache and Pain, vol. 18, no. 1, Feb. 2017, p. 20. PubMed Central, https://doi.org/10.1186/s10194-016-0711-0.
—. “Genetic and Biochemical Changes of the Serotonergic System in Migraine Pathobiology.” The Journal of Headache and Pain, vol. 18, no. 1, Feb. 2017, p. 20. PubMed Central, https://doi.org/10.1186/s10194-016-0711-0.
—. “Genetic and Biochemical Changes of the Serotonergic System in Migraine Pathobiology.” The Journal of Headache and Pain, vol. 18, no. 1, Feb. 2017, p. 20. PubMed Central, https://doi.org/10.1186/s10194-016-0711-0.
Goadsby, Peter J., et al. “Pathophysiology of Migraine: A Disorder of Sensory Processing.” Physiological Reviews, vol. 97, no. 2, Apr. 2017, pp. 553–622. PubMed Central, https://doi.org/10.1152/physrev.00034.2015.
—. “Pathophysiology of Migraine: A Disorder of Sensory Processing.” Physiological Reviews, vol. 97, no. 2, Apr. 2017, pp. 553–622. PubMed Central, https://doi.org/10.1152/physrev.00034.2015.
—. “Pathophysiology of Migraine: A Disorder of Sensory Processing.” Physiological Reviews, vol. 97, no. 2, Apr. 2017, pp. 553–622. PubMed Central, https://doi.org/10.1152/physrev.00034.2015.
Gonçalves, Andre Leite, et al. “Randomised Clinical Trial Comparing Melatonin 3 Mg, Amitriptyline 25 Mg and Placebo for Migraine Prevention.” Journal of Neurology, Neurosurgery, and Psychiatry, vol. 87, no. 10, Oct. 2016, pp. 1127–32. PubMed, https://doi.org/10.1136/jnnp-2016-313458.
Heatley, R. V., et al. “Increased Plasma Histamine Levels in Migraine Patients.” Clinical Allergy, vol. 12, no. 2, Mar. 1982, pp. 145–49. PubMed, https://doi.org/10.1111/j.1365-2222.1982.tb01633.x.
John, P. J., et al. “Effectiveness of Yoga Therapy in the Treatment of Migraine without Aura: A Randomized Controlled Trial.” Headache, vol. 47, no. 5, May 2007, pp. 654–61. PubMed, https://doi.org/10.1111/j.1526-4610.2007.00789.x.
Karimi, Narges, et al. “The Efficacy of Magnesium Oxide and Sodium Valproate in Prevention of Migraine Headache: A Randomized, Controlled, Double-Blind, Crossover Study.” Acta Neurologica Belgica, vol. 121, no. 1, Feb. 2021, pp. 167–73. PubMed, https://doi.org/10.1007/s13760-019-01101-x.
Kayama, Yohei, et al. “Functional Interactions between Transient Receptor Potential M8 and Transient Receptor Potential V1 in the Trigeminal System: Relevance to Migraine Pathophysiology.” Cephalalgia, vol. 38, no. 5, Apr. 2018, pp. 833–45. PubMed Central, https://doi.org/10.1177/0333102417712719.
Key, Felix M., et al. “Human Local Adaptation of the TRPM8 Cold Receptor along a Latitudinal Cline.” PLoS Genetics, vol. 14, no. 5, May 2018, p. e1007298. PubMed, https://doi.org/10.1371/journal.pgen.1007298.
Kilinc, E., et al. “Effects of Nigella Sativa Seeds and Certain Species of Fungi Extracts on Number and Activation of Dural Mast Cells in Rats.” Physiology International, vol. 104, no. 1, Mar. 2017, pp. 15–24. akjournals.com, https://doi.org/10.1556/2060.104.2017.1.8.
Koyuncu Irmak, Duygu, et al. “Shared Fate of Meningeal Mast Cells and Sensory Neurons in Migraine.” Frontiers in Cellular Neuroscience, vol. 13, Apr. 2019, p. 136. PubMed Central, https://doi.org/10.3389/fncel.2019.00136.
Krueger, J. M., et al. “Sleep. A Physiologic Role for IL-1 Beta and TNF-Alpha.” Annals of the New York Academy of Sciences, vol. 856, Sept. 1998, pp. 148–59. PubMed, https://doi.org/10.1111/j.1749-6632.1998.tb08323.x.
Latremoliere, Alban, and Clifford J. Woolf. “Central Sensitization: A Generator of Pain Hypersensitivity by Central Neural Plasticity.” The Journal of Pain : Official Journal of the American Pain Society, vol. 10, no. 9, Sept. 2009, pp. 895–926. PubMed Central, https://doi.org/10.1016/j.jpain.2009.06.012.
Lin, Qi-Fang, et al. “Association of Genetic Loci for Migraine Susceptibility in the She People of China.” The Journal of Headache and Pain, vol. 16, 2015, p. 553. PubMed, https://doi.org/10.1186/s10194-015-0553-1.
Liu, Hua, et al. “Association of 5-HTT Gene Polymorphisms with Migraine: A Systematic Review and Meta-Analysis.” Journal of the Neurological Sciences, vol. 305, no. 1–2, June 2011, pp. 57–66. PubMed, https://doi.org/10.1016/j.jns.2011.03.016.
Liu, Ruozhuo, et al. “MTHFR C677T Polymorphism and Migraine Risk: A Meta-Analysis.” Journal of the Neurological Sciences, vol. 336, no. 1–2, Jan. 2014, pp. 68–73. PubMed, https://doi.org/10.1016/j.jns.2013.10.008.
—. “MTHFR C677T Polymorphism and Migraine Risk: A Meta-Analysis.” Journal of the Neurological Sciences, vol. 336, no. 1–2, Jan. 2014, pp. 68–73. PubMed, https://doi.org/10.1016/j.jns.2013.10.008.
Magalhães, Elza, et al. “Botulinum Toxin Type A versus Amitriptyline for the Treatment of Chronic Daily Migraine.” Clinical Neurology and Neurosurgery, vol. 112, no. 6, July 2010, pp. 463–66. PubMed, https://doi.org/10.1016/j.clineuro.2010.02.004.
Masruha, Marcelo R., et al. “Urinary 6-Sulphatoxymelatonin Levels Are Depressed in Chronic Migraine and Several Comorbidities.” Headache, vol. 50, no. 3, Mar. 2010, pp. 413–19. PubMed, https://doi.org/10.1111/j.1526-4610.2009.01547.x.
McKemy, David D. “TRPM8: The Cold and Menthol Receptor.” TRP Ion Channel Function in Sensory Transduction and Cellular Signaling, edited by Wolfgang B. Liedtke and Stefan Heller, CRC Press/Taylor & Francis, 2007. PubMed, http://www.ncbi.nlm.nih.gov/books/NBK5238/.
—. “TRPM8: The Cold and Menthol Receptor.” TRP Ion Channel Function in Sensory Transduction and Cellular Signaling, edited by Wolfgang B. Liedtke and Stefan Heller, CRC Press/Taylor & Francis, 2007. PubMed, http://www.ncbi.nlm.nih.gov/books/NBK5238/.
Meyer, Melissa M., et al. “Cerebral Sodium (23Na) Magnetic Resonance Imaging in Patients with Migraine – a Case-Control Study.” European Radiology, vol. 29, no. 12, Dec. 2019, pp. 7055–62. PubMed, https://doi.org/10.1007/s00330-019-06299-1.
Meza-Velázquez, R., et al. “Association of Diamine Oxidase and Histamine N-Methyltransferase Polymorphisms with Presence of Migraine in a Group of Mexican Mothers of Children with Allergies.” Neurología (English Edition), vol. 32, no. 8, Oct. 2017, pp. 500–07. www.elsevier.es, https://doi.org/10.1016/j.nrleng.2016.02.012.
“Migraine Aura.” Mayo Clinic, https://www.mayoclinic.org/diseases-conditions/migraine-with-aura/multimedia/migraine-aura/vid-20084707. Accessed 3 Sept. 2021.
“Migraine Intensity, Frequency Linked to High Cholesterol.” National Headache Foundation, 21 Oct. 2015, https://headaches.org/2015/10/21/migraine-intensity-frequency-linked-to-high-cholesterol/.
Mulder, Elles J., et al. “Genetic and Environmental Influences on Migraine: A Twin Study Across Six Countries.” Twin Research and Human Genetics, vol. 6, no. 5, Oct. 2003, pp. 422–31. Cambridge University Press, https://doi.org/10.1375/twin.6.5.422.
Parohan, Mohammad, et al. “The Synergistic Effects of Nano-Curcumin and Coenzyme Q10 Supplementation in Migraine Prophylaxis: A Randomized, Placebo-Controlled, Double-Blind Trial.” Nutritional Neuroscience, vol. 24, no. 4, Apr. 2021, pp. 317–26. PubMed, https://doi.org/10.1080/1028415X.2019.1627770.
Peatfield, R. C. “Relationships between Food, Wine, and Beer-Precipitated Migrainous Headaches.” Headache, vol. 35, no. 6, June 1995, pp. 355–57. PubMed, https://doi.org/10.1111/j.1526-4610.1995.hed3506355.x.
—. “Relationships between Food, Wine, and Beer-Precipitated Migrainous Headaches.” Headache, vol. 35, no. 6, June 1995, pp. 355–57. PubMed, https://doi.org/10.1111/j.1526-4610.1995.hed3506355.x.
Pogoda, Janice M., et al. “Severe Headache or Migraine History Is Inversely Correlated With Dietary Sodium Intake: NHANES 1999-2004.” Headache, vol. 56, no. 4, Apr. 2016, pp. 688–98. PubMed, https://doi.org/10.1111/head.12792.
Ran, Caroline, et al. “Implications for the Migraine SNP Rs1835740 in a Swedish Cluster Headache Population.” The Journal of Headache and Pain, vol. 19, no. 1, Nov. 2018, p. 100. BioMed Central, https://doi.org/10.1186/s10194-018-0937-0.
Rodriguez-Acevedo, Astrid J., et al. “Genetic Association and Gene Expression Studies Suggest That Genetic Variants in the SYNE1 and TNF Genes Are Related to Menstrual Migraine.” The Journal of Headache and Pain, vol. 15, Oct. 2014, p. 62. PubMed, https://doi.org/10.1186/1129-2377-15-62.
Savi, LT, et al. “Prophylaxis of Migraine with Aura: A Place for Acetylsalicylic Acid.” The Journal of Headache and Pain, vol. 14, no. 1, Feb. 2013, p. P195. Springer Link, https://doi.org/10.1186/1129-2377-14-S1-P195.
Sazci, Ali, et al. “Nicotinamide-N-Methyltransferase Gene Rs694539 Variant and Migraine Risk.” The Journal of Headache and Pain, vol. 17, no. 1, Oct. 2016, p. 93. PubMed Central, https://doi.org/10.1186/s10194-016-0688-8.
Schoenen, J., et al. “Effectiveness of High-Dose Riboflavin in Migraine Prophylaxis. A Randomized Controlled Trial.” Neurology, vol. 50, no. 2, Feb. 1998, pp. 466–70. PubMed, https://doi.org/10.1212/wnl.50.2.466.
Schürks, Markus, et al. “A Candidate Gene Association Study of 77 Polymorphisms in Migraine.” The Journal of Pain, vol. 10, no. 7, July 2009, pp. 759–66. PubMed, https://doi.org/10.1016/j.jpain.2009.01.326.
Silva-Néto, R. P., et al. “Odorant Substances That Trigger Headaches in Migraine Patients.” Cephalalgia: An International Journal of Headache, vol. 34, no. 1, Jan. 2014, pp. 14–21. PubMed, https://doi.org/10.1177/0333102413495969.
Sintas, Cèlia, et al. “Replication Study of Previous Migraine Genome-Wide Association Study Findings in a Spanish Sample of Migraine with Aura.” Cephalalgia: An International Journal of Headache, vol. 35, no. 9, Aug. 2015, pp. 776–82. PubMed, https://doi.org/10.1177/0333102414557841.
—. “Replication Study of Previous Migraine Genome-Wide Association Study Findings in a Spanish Sample of Migraine with Aura.” Cephalalgia: An International Journal of Headache, vol. 35, no. 9, Aug. 2015, pp. 776–82. PubMed, https://doi.org/10.1177/0333102414557841.
Stuart, Shani, et al. “The Role of the MTHFR Gene in Migraine.” Headache, vol. 52, no. 3, Mar. 2012, pp. 515–20. PubMed, https://doi.org/10.1111/j.1526-4610.2012.02106.x.
Talebi, Mahnaz, et al. “Relation between Serum Magnesium Level and Migraine Attacks.” Neurosciences (Riyadh, Saudi Arabia), vol. 16, no. 4, Oct. 2011, pp. 320–23.
Terrazzino, Salvatore, et al. “Brain-Derived Neurotrophic Factor Val66Met Gene Polymorphism Impacts on Migraine Susceptibility: A Meta-Analysis of Case–Control Studies.” Frontiers in Neurology, vol. 8, May 2017, p. 159. PubMed Central, https://doi.org/10.3389/fneur.2017.00159.
“Treximet Prices, Coupons & Savings Tips.” GoodRx, https://www.goodrx.com/treximet. Accessed 3 Sept. 2021.
van Oosterhout, Wpj, et al. “Chronotypes and Circadian Timing in Migraine.” Cephalalgia: An International Journal of Headache, vol. 38, no. 4, Apr. 2018, pp. 617–25. PubMed, https://doi.org/10.1177/0333102417698953.
von Luckner, Alexander, and Franz Riederer. “Magnesium in Migraine Prophylaxis-Is There an Evidence-Based Rationale? A Systematic Review.” Headache, vol. 58, no. 2, Feb. 2018, pp. 199–209. PubMed, https://doi.org/10.1111/head.13217.
Wells, Rebecca Erwin, et al. “Meditation for Migraines: A Pilot Randomized Controlled Trial.” Headache, vol. 54, no. 9, Oct. 2014, pp. 1484–95. PubMed, https://doi.org/10.1111/head.12420.
Yılmaz, Ibrahim Arda, et al. “Cytokine Polymorphism in Patients with Migraine: Some Suggestive Clues of Migraine and Inflammation.” Pain Medicine, vol. 11, no. 4, Apr. 2010, pp. 492–97. Silverchair, https://doi.org/10.1111/j.1526-4637.2009.00791.x.
—. “Cytokine Polymorphism in Patients with Migraine: Some Suggestive Clues of Migraine and Inflammation.” Pain Medicine, vol. 11, no. 4, Apr. 2010, pp. 492–97. Silverchair, https://doi.org/10.1111/j.1526-4637.2009.00791.x.
Zhang, X. C., and D. Levy. “Modulation of Meningeal Nociceptors Mechanosensitivity by Peripheral Proteinase-Activated Receptor-2: The Role of Mast Cells.” Cephalalgia: An International Journal of Headache, vol. 28, no. 3, Mar. 2008, pp. 276–84. PubMed, https://doi.org/10.1111/j.1468-2982.2007.01523.x.
Zhang, Xichun, et al. “Activation of Meningeal Nociceptors by Cortical Spreading Depression: Implications for Migraine with Aura.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, vol. 30, no. 26, June 2010, pp. 8807–14. PubMed, https://doi.org/10.1523/JNEUROSCI.0511-10.2010.