Key takeaways:
~Mutations in the SERPINA1 gene causes alpha-1 antitrypsin deficiency.
~ Alpha-1 antitrypsin (AAT) deficiency increases susceptibility to respiratory issues, COPD (chronic obstructive pulmonary disease), and in some cases, liver dysfunction.
~ Alpha-1 antitrypsin also interacts with mast cells, affects the pancreas, and modulates immune response.
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Consider joining today.
What is alpha-1 antitrypsin?
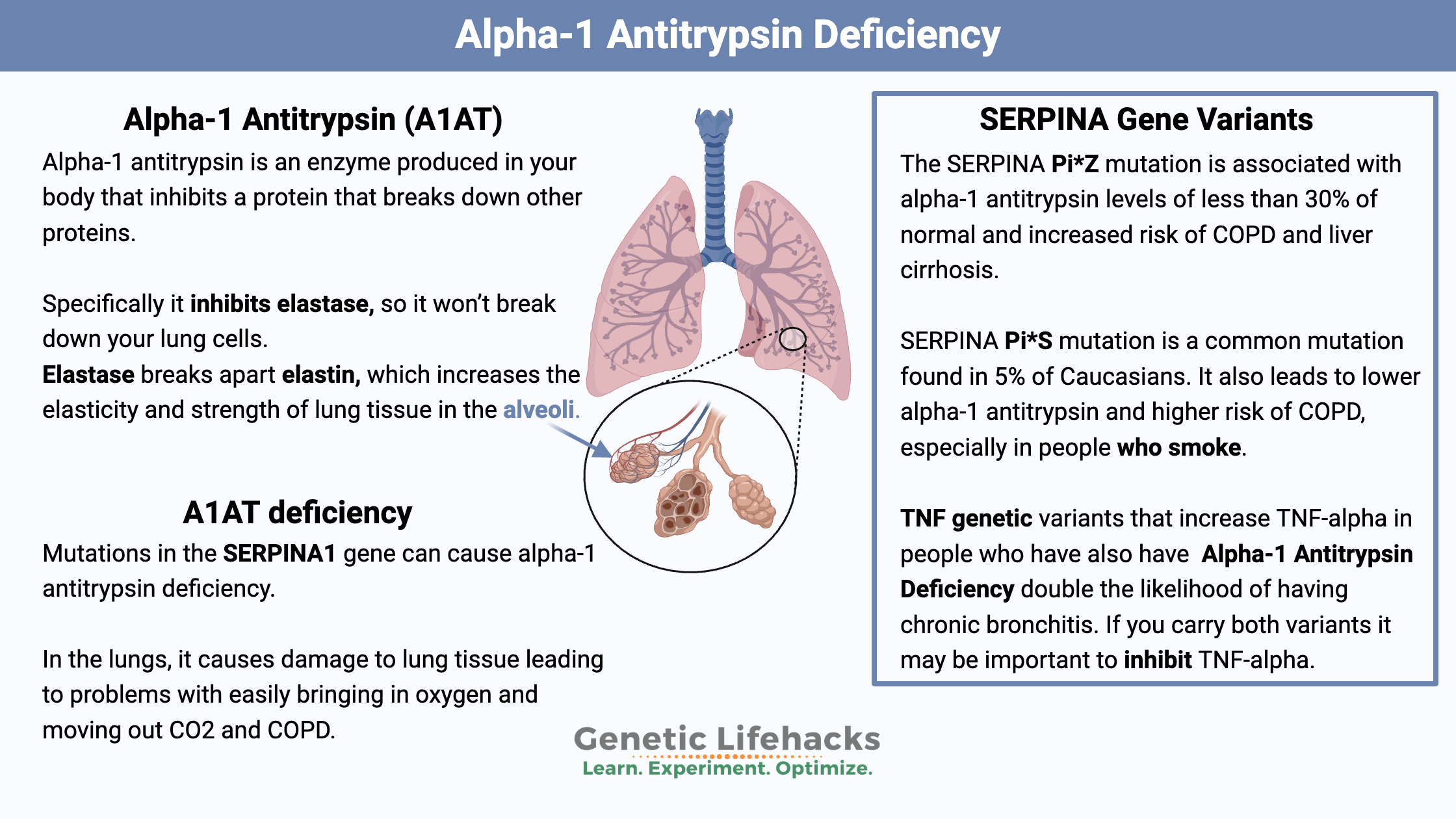
Alpha-1 antitrypsin is an enzyme produced in your body. The SERPINA1 (serine protease inhibitor 1) gene codes for the alpha-1 antitrypsin protein. Alpha-1 antitrypsin was named a while ago – before the protein’s function was fully understood – so its name is a bit misleading.
Elastin and Elastase:
Specific cells in your lungs produce a protein called elastin. The elastin increases the elasticity and strength of the lung tissue in the alveoli, which are the tiny air sacs where oxygen is exchanged with carbon dioxide.
Elastin, though, isn’t unique just to your lung cells. Gram-negative bacteria also produce the elastin protein. As a result, your immune system (specifically the neutrophils) creates an enzyme called elastase to break apart the elastin in the outer membrane of the bacteria.
Elastase is great – when you need to get rid of bacteria. But you don’t want too much elastase hanging around because it can also break down the elastin in the cells in your lungs.
Alpha-1 antitrypsin comes into play here. It is a protease inhibitor – meaning it inhibits a protein that breaks down other proteins. Specifically, alpha-1 antitrypsin inhibits elastase, so it won’t break down your lung cells.
Alpha-1 antitrypsin is predominately made in the liver and then transported to the lungs. In the lungs, it deactivates elastase before it damages lung cells.
Pretty neat system! Elastase can be used by neutrophils to attack gram-negative bacteria in the lungs, but alpha-1 antitrypsin keeps it from damaging your lung cells.
Elastase – beyond lung cells:
Elastase is also essential in other body areas, such as the skin and blood vessels.[ref] One important place is the blood-brain barrier.[ref]
Other enzymes inhibited by alpha-1 antitrypsin:
The immune system response is a balancing act, and alpha-1 antitrypsin plays a role in preventing damage from our immune response.
While the focus of much of the research over the last half-century has been on elastase and lung function, alpha-1 antitrypsin also inhibits proteinase 2, trypsin, cathepsin G, and other enzyme factors.[ref]
Additionally, alpha-1 antitrypsin neutralizes chymase and tryptase from mast cells.
In the pancreas, alpha-1 antitrypsin protects beta cells from cell death due to caspase 3 (important in type 1 diabetes).[ref]
Inflammation, the immune system, and alpha-1 antitrypsin:
Alpha-1 antitrypsin has also been shown to act as an anti-inflammatory molecule, inhibiting neutrophil superoxide production, preventing cell death in liver cells, and inhibiting monocyte activation.[ref]
Inflammation increases the liver’s production of alpha-1 antitrypsin. As neutrophils release elastase to combat bacterial infections, there is a response by the liver to increase the alpha-1 antitrypsin enzyme.[ref]
Other cells can also create the alpha-1 antitrypsin protein, and tissues can produce it in the local response to inflammation.[ref][ref]
In the acute-phase response — the immune response immediately after an injury or infection — alpha-1 antitrypsin production increases four-fold. Alpha-1 anti-tryptase is activated by lipopolysaccharide (present on gram-negative bacteria), TNF-alpha, oxidative stress, IL-1, and IL-6.[ref]
Interestingly, alpha-1 antitrypsin plasma infusions (via IV) have shown to be effective for some people with autoimmune disorders (type 1 diabetes, Crohn’s, MS, fibromyalgia, RA) and chronic fatigue syndrome (ME/CFS).[ref]
COVID-19: Although lacking in clinical data, there are several reports that alpha-1 antitrypsin deficiency may increase the risk of severe COVID-19. Talk with your doctor and read through these references… [ref][ref][ref]
What is alpha-1 antitrypsin (A1AT) deficiency?
Alpha-1 antitrypsin deficiency is considered one of the most common hereditary diseases worldwide.
Certain mutations in the SERPINA1 gene can cause alpha-1 antitrypsin deficiency due to the alpha-1 antitrypsin protein not functioning appropriately.[ref]
In the lungs:
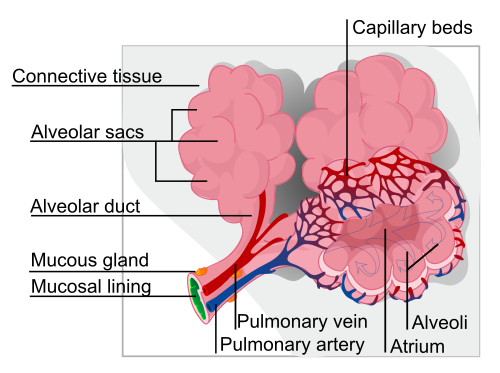
Without alpha-1, there can be too much elastase, causing damage to lung tissue by breaking down elastin. The damage occurs in the alveoli, the little sacs vital for exchanging oxygen and carbon dioxide.
When the alveoli lose some of their elasticity, it can cause problems with easily bringing in oxygen and moving out CO2.
Thus, people who carry alpha-1 antitrypsin deficiency mutations are more likely to have COPD – chronic obstructive pulmonary disease.
COPD causes shortness of breath, wheezing, cough, and mucus production. Other terms for COPD include emphysema and chronic bronchitis.
As you can imagine, smoking is terrible for people with alpha-1 antitrypsin deficiency. Carriers of alpha-1 antitrypsin deficiency mutations are at a much higher and earlier risk of COPD compared with smokers without the mutation.
In the liver:
The liver produces alpha-1 antitrypsin in response to signals from the body for illness (fever, inflammatory signals). Therefore, it counteracts the neutrophil’s production of elastase at a time when the neutrophils are actively combating an infection.[ref]
The folding of the alpha-1 antitrypsin protein can be affected by the SERPINA1 mutations. The misfolded protein can then get stuck in the liver, unable to be transported to the lungs. This can cause liver damage (in addition to lacking the enzyme in the lungs).
Heterozygous advantage: Taller with greater lung volume as a baby
When mutations that cause a genetic disease are much more common in the population than they statistically should be, researchers often check to see if there is an advantage for people who carry one copy of the mutation. For example, people who carry one copy of the sickle cell anemia mutation are more resistant to malaria. The sickle cell mutation is more commonly found in people who live in Africa, where malaria is highly prevalent. People who carry one copy of the sickle cell mutation are more likely to survive malaria, thus passing on the mutation to their children.
A study published in 2016 looked at several different genetic diseases to see if there was an advantage to carrying one copy of the mutation. For alpha-1 antitrypsin deficiency, the researchers found that carriers of one copy of the Pi*Z mutation tended to be taller (1.5cm on average) and had greater initial lung volume. This finding could lead to a survival advantage for babies, thus passing the mutation to offspring.[ref]
Alpha-1 Antitrypsin Genotype Report:
Interactions with other diseases:
Hemochromatosis mutations:
With the increase in iron and ferritin levels from SERPINA1 mutations, you should also check to see if you carry mutations in the HFE gene that can cause hemochromatosis (iron overload): Building Up Iron: Check your genetic data for hemochromatosis mutations
Fatty liver disease:
People who carry the SERPINA1 mutations are at a higher risk of liver problems in conjunction with fatty liver disease (NAFLD). With fatty liver disease occurring now in an estimated half of the US population, this is something to consider if you carry one of the SERPINA1 mutations. Check your genes for other fatty liver disease risk factors: Fatty Liver: Genetic variants that increase the risk of NAFLD.
Cystic Fibrosis:
Carrying a SERPINA1 mutation increases the risk of liver disease in cystic fibrosis patients. Check your genetic data for cystic fibrosis mutations.
Lifehacks:
Testing:
Your doctor can run tests to see what your alpha-1 trypsin levels are. If you carry two copies of the mutation, you should talk with your doctor about testing and possible long-term lung and liver function implications.
If you currently don’t have a doctor, or if your doctor won’t order it, you can order the alpha-1 antitrypsin test yourself from online lab companies and then get your blood drawn locally. For example, Ulta Lab Tests offers it for $49. (Shop around, lab test costs online vary a lot)
There are plasma AAT therapies available for alpha-1 antitrypsin deficiency, which your doctor can prescribe if needed.[ref]
If you wonder what the serum levels of alpha-1 antitrypsin should be, check out Table 2 in this article.
Lifestyle:
This mutation is one where it would be an excellent idea not to drink or smoke.
Additionally, occupations that expose you to a lot of airborne particulate matter are not a good idea…
Here are a couple of excellent resources for more information:
Inflammation, TNF-alpha interaction:
Related Articles and Topics:
TNF-alpha: Inflammation and Your Genes
Do you feel like you are always dealing with inflammation? Joint pain, food sensitivity, etc.? Perhaps you are genetically geared towards a higher inflammatory response. Tumor necrosis factor (TNF) is an inflammatory cytokine that acts as a signaling molecule in our immune system. In an acute inflammatory situation, TNF-alpha plays an essential role in protecting us.
Fatty Liver: Genetic variants that increase the risk of NAFLD
Non-alcoholic fatty liver disease (NAFLD) is now the leading cause of liver problems worldwide, bypassing alcoholic liver disease. It is estimated that almost half of the population in the US has NAFLD caused by a combination of genetic susceptibility, diet, and lifestyle factors.
CTLA-4: Autoimmune Genetic Risk
The CTLA4 gene codes for a protein that is important in the immune system. It acts as a checkpoint that can downregulate your immune system response. Genetic variants in the CTLA4 gene can increase your risk for several different autoimmune diseases.
References:
Abbas, Amr M., and Hussein F. Sakr. “Effect of Magnesium Sulfate and Thyroxine on Inflammatory Markers in a Rat Model of Hypothyroidism.” Canadian Journal of Physiology and Pharmacology, vol. 94, no. 4, Apr. 2016, pp. 426–32. PubMed, https://doi.org/10.1139/cjpp-2015-0247.
Bagchi, Sounak, et al. “In-Vitro Blood-Brain Barrier Models for Drug Screening and Permeation Studies: An Overview.” Drug Design, Development and Therapy, vol. 13, Oct. 2019, pp. 3591–605. PubMed Central, https://doi.org/10.2147/DDDT.S218708.
Basyte-Bacevice, Viktorija, et al. “SERPINA1 and HSD17B13 Gene Variants in Patients with Liver Fibrosis and Cirrhosis.” Journal of Gastrointestinal and Liver Diseases: JGLD, vol. 28, no. 3, Sept. 2019, pp. 297–302. PubMed, https://doi.org/10.15403/jgld-168.
Blancas-Flores, Gerardo, et al. “Glycine Suppresses TNF-α-Induced Activation of NF-ΚB in Differentiated 3T3-L1 Adipocytes.” European Journal of Pharmacology, vol. 689, no. 1–3, Aug. 2012, pp. 270–77. PubMed, https://doi.org/10.1016/j.ejphar.2012.06.025.
Bonkovsky, Herbert L., et al. “Genetic Polymorphisms Implicated in Nonalcoholic Liver Disease or Selected Other Disorders Have No Influence on Drug‐Induced Liver Injury.” Hepatology Communications, vol. 3, no. 8, June 2019, pp. 1032–35. PubMed Central, https://doi.org/10.1002/hep4.1382.
Borthakur, Alip, et al. “The Probiotic Lactobacillus Plantarum Counteracts TNF-{alpha}-Induced Downregulation of SMCT1 Expression and Function.” American Journal of Physiology. Gastrointestinal and Liver Physiology, vol. 299, no. 4, Oct. 2010, pp. G928-934. PubMed, https://doi.org/10.1152/ajpgi.00279.2010.
Csiszar, Anna, Kira Smith, et al. “Resveratrol Attenuates TNF-Alpha-Induced Activation of Coronary Arterial Endothelial Cells: Role of NF-KappaB Inhibition.” American Journal of Physiology. Heart and Circulatory Physiology, vol. 291, no. 4, Oct. 2006, pp. H1694-1699. PubMed, https://doi.org/10.1152/ajpheart.00340.2006.
Csiszar, Anna, Nazar Labinskyy, et al. “Vasoprotective Effects of Resveratrol and SIRT1: Attenuation of Cigarette Smoke-Induced Oxidative Stress and Proinflammatory Phenotypic Alterations.” American Journal of Physiology. Heart and Circulatory Physiology, vol. 294, no. 6, June 2008, pp. H2721–35. PubMed Central, https://doi.org/10.1152/ajpheart.00235.2008.
Curjuric, Ivan, et al. “Alpha-1 Antitrypsin Deficiency: From the Lung to the Heart?” Atherosclerosis, vol. 270, Mar. 2018, pp. 166–72. PubMed, https://doi.org/10.1016/j.atherosclerosis.2018.01.042.
de Serres, F., and I. Blanco. “Role of Alpha-1 Antitrypsin in Human Health and Disease.” Journal of Internal Medicine, vol. 276, no. 4, Oct. 2014, pp. 311–35. DOI.org (Crossref), https://doi.org/10.1111/joim.12239.
de Serres, Frederick J. “Worldwide Racial and Ethnic Distribution of Alpha1-Antitrypsin Deficiency: Summary of an Analysis of Published Genetic Epidemiologic Surveys.” Chest, vol. 122, no. 5, Nov. 2002, pp. 1818–29. PubMed, https://doi.org/10.1378/chest.122.5.1818.
Ehlers, Mario R. “Immune-Modulating Effects of Alpha-1 Antitrypsin.” Biological Chemistry, vol. 395, no. 10, Oct. 2014, pp. 1187–93. PubMed Central, https://doi.org/10.1515/hsz-2014-0161.
Ferrarotti, Ilaria, et al. “Identification and Characterisation of Eight Novel SERPINA1 Null Mutations.” Orphanet Journal of Rare Diseases, vol. 9, Nov. 2014, p. 172. PubMed Central, https://doi.org/10.1186/s13023-014-0172-y.
Fregonese, Laura, and Jan Stolk. “Hereditary Alpha-1-Antitrypsin Deficiency and Its Clinical Consequences.” Orphanet Journal of Rare Diseases, vol. 3, no. 1, June 2008, p. 16. Springer Link, https://doi.org/10.1186/1750-1172-3-16.
Guldiken, Nurdan, et al. “Mild Iron Overload as Seen in Individuals Homozygous for the Alpha-1 Antitrypsin Pi*Z Variant Does Not Promote Liver Fibrogenesis in HFE Knockout Mice.” Cells, vol. 8, no. 11, Nov. 2019, p. E1415. PubMed, https://doi.org/10.3390/cells8111415.
Guo, Ying, et al. “Prophylactic Effects of Bifidobacterium Adolescentis on Anxiety and Depression-Like Phenotypes After Chronic Stress: A Role of the Gut Microbiota-Inflammation Axis.” Frontiers in Behavioral Neuroscience, vol. 13, 2019, p. 126. PubMed, https://doi.org/10.3389/fnbeh.2019.00126.
Janciauskiene, Sabina M., et al. “The Discovery of Α1-Antitrypsin and Its Role in Health and Disease.” Respiratory Medicine, vol. 105, no. 8, Aug. 2011, pp. 1129–39. PubMed, https://doi.org/10.1016/j.rmed.2011.02.002.
Kim, Dong-Gyu, et al. “Antiinflammatory Effects of Functionally Active Compounds Isolated from Aged Black Garlic.” Phytotherapy Research: PTR, vol. 31, no. 1, Jan. 2017, pp. 53–61. PubMed, https://doi.org/10.1002/ptr.5726.
Klemenak, Martina, et al. “Administration of Bifidobacterium Breve Decreases the Production of TNF-α in Children with Celiac Disease.” Digestive Diseases and Sciences, vol. 60, no. 11, Nov. 2015, pp. 3386–92. PubMed, https://doi.org/10.1007/s10620-015-3769-7.
Knoell, D. L., et al. “Alpha 1-Antitrypsin and Protease Complexation Is Induced by Lipopolysaccharide, Interleukin-1beta, and Tumor Necrosis Factor-Alpha in Monocytes.” American Journal of Respiratory and Critical Care Medicine, vol. 157, no. 1, Jan. 1998, pp. 246–55. PubMed, https://doi.org/10.1164/ajrccm.157.1.9702033.
Morihara, Naoaki, et al. “Aged Garlic Extract Suppresses the Development of Atherosclerosis in Apolipoprotein E-Knockout Mice.” The Journal of Nutrition, vol. 146, no. 2, Feb. 2016, pp. 460S-463S. PubMed, https://doi.org/10.3945/jn.114.206953.
Nam, Bora, et al. “Lactobacillus Plantarum HY7714 Restores TNF-α Induced Defects on Tight Junctions.” Preventive Nutrition and Food Science, vol. 24, no. 1, Mar. 2019, pp. 64–69. PubMed, https://doi.org/10.3746/pnf.2019.24.1.64.
NM_000295.5(SERPINA1):C.1177C>A (p.Pro393Thr) AND Alpha-1-Antitrypsin Deficiency – ClinVar – NCBI. https://www.ncbi.nlm.nih.gov/clinvar/RCV000206411.1/. Accessed 11 May 2022.
North, Teri-Louise, et al. “A Study of Common Mendelian Disease Carriers across Ageing British Cohorts: Meta-Analyses Reveal Heterozygosity for Alpha 1-Antitrypsin Deficiency Increases Respiratory Capacity and Height.” Journal of Medical Genetics, vol. 53, no. 4, Apr. 2016, pp. 280–88. PubMed Central, https://doi.org/10.1136/jmedgenet-2015-103342.
Ortega, Victor E., et al. “The Effects of Rare SERPINA1 Variants on Lung Function and Emphysema in SPIROMICS.” American Journal of Respiratory and Critical Care Medicine, vol. 201, no. 5, Mar. 2020, pp. 540–54. PubMed, https://doi.org/10.1164/rccm.201904-0769OC.
Petrache, Irina, et al. “Safety and Efficacy of Alpha-1-Antitrypsin Augmentation Therapy in the Treatment of Patients with Alpha-1-Antitrypsin Deficiency.” Biologics: Targets & Therapy, vol. 3, 2009, pp. 193–204. PubMed, https://doi.org/10.2147/btt.2009.3088.
Prados, M., et al. “Phenotypes of Alpha-1-Antitrypsin in Intrinsic Asthma and ASA-Triad Patients.” Allergologia Et Immunopathologia, vol. 23, no. 1, Feb. 1995, pp. 24–28.
Rodriguez, Francisco, et al. “Glutathione S-Transferase P1 and Lung Function in Patients with Alpha1-Antitrypsin Deficiency and COPD.” Chest, vol. 127, no. 5, May 2005, pp. 1537–43. PubMed, https://doi.org/10.1378/chest.127.5.1537.
Rudnick, David A., et al. “Indomethacin Increases Liver Damage in a Murine Model of Liver Injury from Alpha-1-Antitrypsin Deficiency.” Hepatology (Baltimore, Md.), vol. 44, no. 4, Oct. 2006, pp. 976–82. PubMed, https://doi.org/10.1002/hep.21326.
Rui, Yehua, et al. “Rosmarinic Acid Suppresses Adipogenesis, Lipolysis in 3T3-L1 Adipocytes, Lipopolysaccharide-Stimulated Tumor Necrosis Factor-α Secretion in Macrophages, and Inflammatory Mediators in 3T3-L1 Adipocytes.” Food & Nutrition Research, vol. 61, no. 1, 2017, p. 1330096. PubMed, https://doi.org/10.1080/16546628.2017.1330096.
Shao, Nan, et al. “Curcumin Improves Treatment Outcome of Takayasu Arteritis Patients by Reducing TNF-α: A Randomized Placebo-Controlled Double-Blind Clinical Trial.” Immunologic Research, vol. 65, no. 4, Aug. 2017, pp. 969–74. PubMed, https://doi.org/10.1007/s12026-017-8917-z.
Song, Sihong. “Alpha-1 Antitrypsin Therapy for Autoimmune Disorders.” Chronic Obstructive Pulmonary Diseases: Journal of the COPD Foundation, vol. 5, no. 4, pp. 289–301. PubMed Central, https://doi.org/10.15326/jcopdf.5.4.2018.0131. Accessed 11 May 2022.
Strnad, Pavel, et al. “Heterozygous Carriage of the Alpha1-Antitrypsin Pi*Z Variant Increases the Risk to Develop Liver Cirrhosis.” Gut, vol. 68, no. 6, June 2019, pp. 1099–107. PubMed, https://doi.org/10.1136/gutjnl-2018-316228.
Thun, Gian Andri, et al. “Causal and Synthetic Associations of Variants in the SERPINA Gene Cluster with Alpha1-Antitrypsin Serum Levels.” PLoS Genetics, vol. 9, no. 8, Aug. 2013, p. e1003585. PubMed Central, https://doi.org/10.1371/journal.pgen.1003585.
Thun, Gian-Andri, et al. “SERPINA1 PiZ and PiS Heterozygotes and Lung Function Decline in the SAPALDIA Cohort.” PloS One, vol. 7, no. 8, 2012, p. e42728. PubMed, https://doi.org/10.1371/journal.pone.0042728.
Tsilioni, I., et al. “Children with Autism Spectrum Disorders, Who Improved with a Luteolin-Containing Dietary Formulation, Show Reduced Serum Levels of TNF and IL-6.” Translational Psychiatry, vol. 5, no. 9, Sept. 2015, p. e647. PubMed Central, https://doi.org/10.1038/tp.2015.142.
VCV000017966.2 – ClinVar – NCBI. https://www.ncbi.nlm.nih.gov/clinvar/variation/17966/. Accessed 11 May 2022.
Wettstein, Lukas, Carina Conzelmann, et al. Alpha-1 Antitrypsin Inhibits SARS-CoV-2 Infection. bioRxiv, 2 July 2020, p. 2020.07.02.183764. bioRxiv, https://www.biorxiv.org/content/10.1101/2020.07.02.183764v1.
Wettstein, Lukas, Tatjana Weil, et al. “Alpha-1 Antitrypsin Inhibits TMPRSS2 Protease Activity and SARS-CoV-2 Infection.” Nature Communications, vol. 12, no. 1, Mar. 2021, p. 1726. www.nature.com, https://doi.org/10.1038/s41467-021-21972-0.
“What Is Alpha-1.” Alpha-1 Foundation, https://www.alpha1.org/what-is-alpha1/. Accessed 11 May 2022.
Wood, Alice M., et al. “The TNFalpha Gene Relates to Clinical Phenotype in Alpha-1-Antitrypsin Deficiency.” Respiratory Research, vol. 9, no. 1, 2008, p. 52. PubMed Central, https://doi.org/10.1186/1465-9921-9-52.
Xu, Ting, et al. “Oral Application of Magnesium-L-Threonate Attenuates Vincristine-Induced Allodynia and Hyperalgesia by Normalization of Tumor Necrosis Factor-α/Nuclear Factor-ΚB Signaling.” Anesthesiology, vol. 126, no. 6, June 2017, pp. 1151–68. PubMed, https://doi.org/10.1097/ALN.0000000000001601.
Yang, Chengliang, et al. “Α1-Antitrypsin Deficiency and the Risk of COVID-19: An Urgent Call to Action.” The Lancet Respiratory Medicine, vol. 9, no. 4, Apr. 2021, pp. 337–39. www.thelancet.com, https://doi.org/10.1016/S2213-2600(21)00018-7.
Zaharuddin, Liyana, et al. “A Randomized Double-Blind Placebo-Controlled Trial of Probiotics in Post-Surgical Colorectal Cancer.” BMC Gastroenterology, vol. 19, no. 1, July 2019, p. 131. PubMed, https://doi.org/10.1186/s12876-019-1047-4.
Originally published 12/2019, updated 2/2021