Key takeaways:
~ Mercury exposure has long been known to cause neurological problems.
~ Organic mercury is more likely to cause health issues than inorganic mercury.
~ Genetic variants play a role in how quickly you excrete mercury; diet and lifestyle factors are also important.
~ Research studies show that certain natural supplements may help with detoxification.
Mercury: Effects and Detoxification
Mercury is the only metal that is a liquid at normal temperatures and pressures. It is classified as a heavy metal (along with lead, cadmium, iron, zinc, etc.), and it can be toxic in the body even at very low levels.
Mercury can build up in the body, causing negative health effects on the central nervous system and the kidneys. It is a fancy way of saying that it hurts the brain, causing fatigue, memory impairments, nervousness, insomnia, and tremor. Exposure to a lot of mercury at once can cause nausea, vomiting, kidney damage, bloody diarrhea, and even death.[ref]
Exposure routes: how does mercury get in the body
We are exposed to mercury through a variety of pathways:
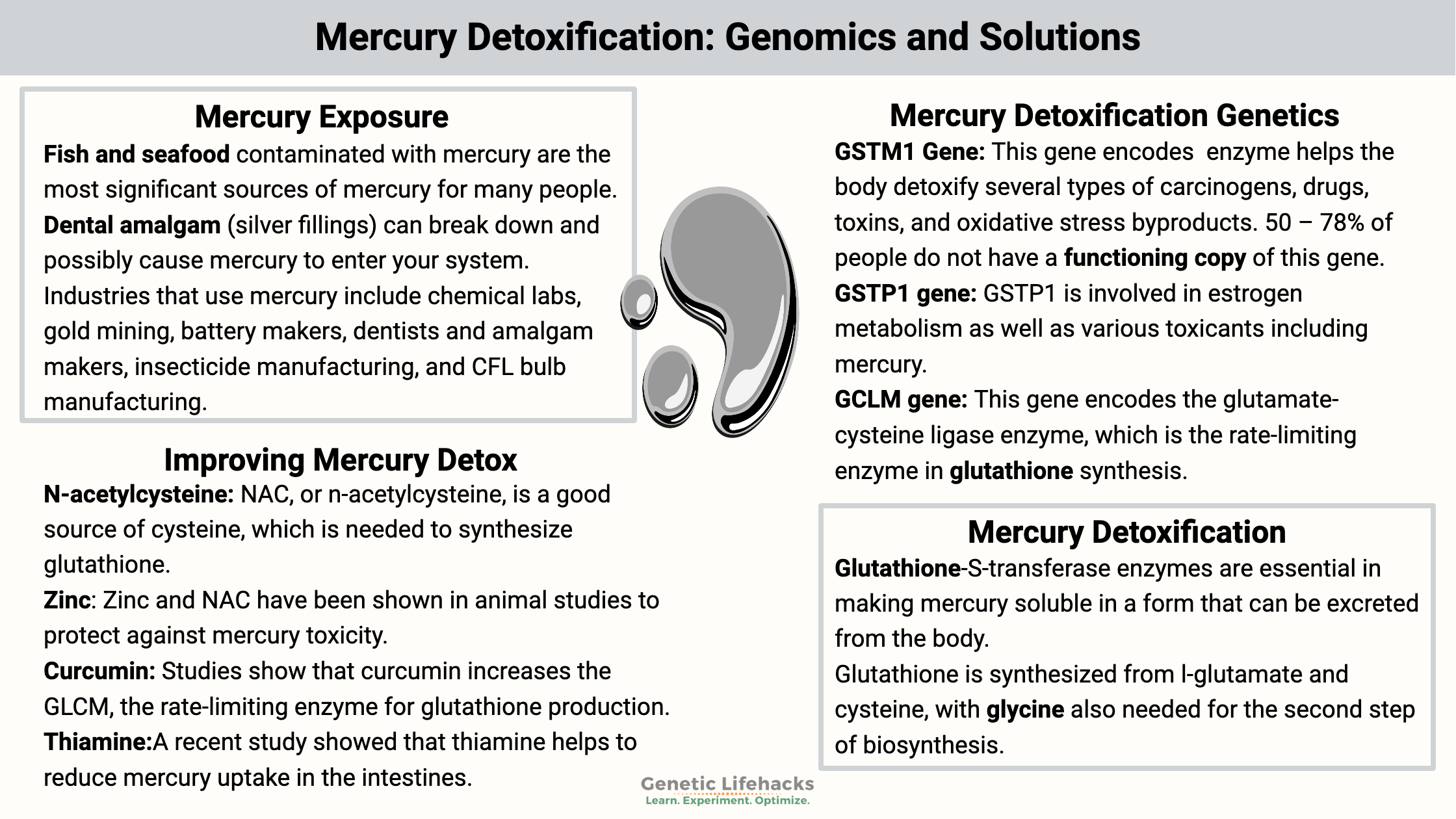
- Fish and seafood contaminated with mercury are the most significant sources of mercury for many people.
- Dental amalgam (silver fillings) can break down and possibly cause mercury to enter your system.
- Certain vaccines contain trace amounts of mercury (thimerosal) as a preservative.
- Occupational exposure can happen either through breathing in mercury-contaminated air or via skin exposure. Industries that use mercury include chemical labs, gold mining, battery makers, dentists and amalgam makers, insecticide manufacturing, and CFL bulb manufacturing.
Air pollution containing mercury can also be a problem in some areas. Elemental mercury can be a contaminant in air pollution, and it also can be inhaled when near liquid mercury. Liquid mercury (such as in CFL bulbs or thermometers) easily vaporizes and is absorbed in the lungs. Mercury can also be absorbed through the skin.
Forms of mercury:
All forms of mercury can be a problem, depending on the concentration. When it comes to detoxifying mercury, though, the different forms of mercury use different detox pathways.
Mercury can be classified as:
- Organic mercury: mainly methylmercury, which is the form found in seafood
- Inorganic mercury: elemental mercury in air pollution (fossil fuel emissions), evaporation from liquid mercury, mercuric salts dissolved in water
Organic mercury is formed from inorganic, airborne mercury, such as from fossil fuel emissions entering bodies of water. In aquatic sediments, certain bacteria convert mercury into methylmercury. Extremely toxic, methylmercury can be easily absorbed via drinking water or eating fish that have absorbed the methylmercury.
Dental amalgams – silver fillings – are another source of exposure to inorganic mercury. To add to the complexity, some forms of mercury convert in vivo from inorganic to organic.
Why is mercury toxic?
While mercury toxicity can affect someone at any age, it is especially devastating to a developing fetus. Exposure to low levels of mercury can affect brain formation by killing off the neuronal stem cells that form the brain.
Mercury also affects children and adult brains, just not at the devastating level seen in developing babies. Neurogenesis, or the formation of new neurons, happens at low levels throughout life. Research shows that mercury inhibits the development of astrocytes from neuronal progenitor cells. Instead, there is a shift in cell type from astrocyte formation to more glial cells. While both cell types are important, we need them in the right amount. Essentially, methylmercury is thought to disrupt some of the cell signaling taking place when neurons are forming.[ref]
The term “Mad as a hatter” referred to the neurological changes from long-term mercury exposure. In England, the process of making felt hats used to include using mercury to stabilize the wool. Hat makers often ended up with mercury poisoning symptoms that caused memory loss and personality changes.
Mercury is stored in the brain at a higher level than the rest of the body. Some studies show that the brain stores mercury at 3 to 6 times the rate of the rest of the body.[ref]
Recent research points to a potentially significant way that mercury causes toxicity. Studies now show that mercury can take the place of selenium in specific proteins, causing those proteins not to function. The selenoproteins that are targeted include thioredoxin reductase 1 and 2. These are major antioxidant and redox-regulating enzymes.[ref]
How long does mercury stay in the body?
When inhaled, organic mercury has a half-life of around 60 days in the body. But mercury in the brain can stay there for 20 years or more.[ref]
It takes time for the symptoms of mercury toxicity to show up. Research shows that it can take up to 5 months for some symptoms to appear.[ref]
How is mercury detoxified and excreted?
Mercury is eliminated primarily by glutathione detoxification. It is excreted in bile, feces, or urine. The glutathione-S-transferase enzymes are essential in making mercury soluble in a form that can be excreted from the body.[ref]
Glutathione is synthesized from l-glutamate and cysteine, with glycine also needed for the second step of biosynthesis. All animal cells can synthesize glutathione, and it is an essential antioxidant and detoxification compound.
Related article: Glutathione: antioxidant, detoxification:
With the help of enzymes to catalyze the reaction, glutathione is bound to methylmercury (the kind found in fish) for excretion. There are also a couple of other routes of detoxification of mercury. For elemental mercury, free cysteine is also utilized for detoxification. Metallothioneins are cysteine-rich proteins that can bind with mercury for excretion in certain circumstances.[ref]
We want mercury to be excreted as quickly as possible so that it doesn’t hang around and end up in the brain. This detoxification pathway brings us to the genetic variants that impact how well your body excretes mercury.
Mercury Detoxification Genotype Report:
Lifehacks:
Obviously, you want to avoid exposure to mercury at higher levels. The fact that it can remain in the brain for 20 years is disconcerting.
Which types of fish are highest in mercury?
According to the FDA, fish high in mercury include (listed higher to lower):[ref]
- tilefish
- swordfish
- shark
- king mackerel
- tuna
- orange roughy
- marlin
- grouper
- Chilean bass
- tuna (canned, Albacore)
- white croaker
- halibut
Drink tea with your fish? A recent study showed that tannic acid and EGCG, found in black or green tea, help to block mercury absorption from foods.[ref]
Testing for mercury:
Blood tests and hair metal tests are available to check for mercury. Mercury accumulates in the hair at about 250x the amount seen in the blood, on average. Hair testing accuracy depends on age, gender, and genetics. Normal levels of mercury in the hair are 1-2 ug/g. In hair testing for adults, neurological effects are seen at 50 ug/g. In blood mercury tests, people who don’t eat fish usually have levels below 2ug/L, while individuals who consume a lot of contaminated fish may range from 5 to 10ug/L.[ref]
Supplements that may help with mercury detoxification:
Related Articles and Topics:
Magnesium and Your Genes
Learn how genomics impacts the need for magnesium.
Inflammation: Causes and Natural Solution
Take a deep dive into the causes of chronic inflammation and learn how to target specific inflammatory pathways to reverse or prevent chronic disease.
GSTs: glutathione-S-transferase enzymes for detoxifying environmental toxins.
Exposure to many different man-made chemical compounds occurs every day, and our exposure to new toxicants well exceeds what our ancestors experienced. Several common GST variants decrease the function of the GST enzymes.
SOD1: Genetic Variants in Our Antioxidant Defense System
Our body has built-in antioxidants that fight against cellular stress. The superoxide dismutase enzyme fights against oxidative stress in your cells.
References:
Abbott, Louise C., and Fikru Nigussie. “Mercury Toxicity and Neurogenesis in the Mammalian Brain.” International Journal of Molecular Sciences, vol. 22, no. 14, July 2021, p. 7520. PubMed Central, https://doi.org/10.3390/ijms22147520.
Abu-Taweel, Gasem Mohammad. “Neurobehavioral Protective Properties of Curcumin against the Mercury Chloride Treated Mice Offspring.” Saudi Journal of Biological Sciences, vol. 26, no. 4, May 2019, pp. 736–43. PubMed, https://doi.org/10.1016/j.sjbs.2018.10.016.
Andreoli, Virginia, and Francesca Sprovieri. “Genetic Aspects of Susceptibility to Mercury Toxicity: An Overview.” International Journal of Environmental Research and Public Health, vol. 14, no. 1, Jan. 2017, p. 93. PubMed Central, https://doi.org/10.3390/ijerph14010093.
Ballatori, N., et al. “N-Acetylcysteine as an Antidote in Methylmercury Poisoning.” Environmental Health Perspectives, vol. 106, no. 5, May 1998, pp. 267–71. PubMed, https://doi.org/10.1289/ehp.98106267.
Barcelos, Gustavo Rafael Mazzaron, et al. “Polymorphisms in Glutathione-Related Genes Modify Mercury Concentrations and Antioxidant Status in Subjects Environmentally Exposed to Methylmercury.” The Science of the Total Environment, vol. 463–464, Oct. 2013, pp. 319–25. PubMed, https://doi.org/10.1016/j.scitotenv.2013.06.029.
Branco, Vasco, et al. “Biomarkers of Mercury Toxicity: Past, Present and Future Trends.” Journal of Toxicology and Environmental Health. Part B, Critical Reviews, vol. 20, no. 3, 2017, pp. 119–54. PubMed Central, https://doi.org/10.1080/10937404.2017.1289834.
Clarkson, Thomas W., and Laszlo Magos. “The Toxicology of Mercury and Its Chemical Compounds.” Critical Reviews in Toxicology, vol. 36, no. 8, Sept. 2006, pp. 609–62. PubMed, https://doi.org/10.1080/10408440600845619.
Custodio, Hipolito M., Raul Harari, et al. “Genetic Influences on the Retention of Inorganic Mercury.” Archives of Environmental & Occupational Health, vol. 60, no. 1, Feb. 2005, pp. 17–23. PubMed, https://doi.org/10.3200/AEOH.60.1.17-23.
Custodio, Hipolito M., Karin Broberg, et al. “Polymorphisms in Glutathione-Related Genes Affect Methylmercury Retention.” Archives of Environmental Health, vol. 59, no. 11, Nov. 2004, pp. 588–95. PubMed, https://doi.org/10.1080/00039890409603438.
de Oliveira, Andréia Ávila Soares, et al. “Genetic Polymorphisms in Glutathione (GSH-) Related Genes Affect the Plasmatic Hg/Whole Blood Hg Partitioning and the Distribution between Inorganic and Methylmercury Levels in Plasma Collected from a Fish-Eating Population.” BioMed Research International, vol. 2014, 2014, p. 940952. PubMed Central, https://doi.org/10.1155/2014/940952.
Engström, Karin Schläwicke, et al. “Genetic Variation in Glutathione-Related Genes and Body Burden of Methylmercury.” Environmental Health Perspectives, vol. 116, no. 6, June 2008, pp. 734–39. PubMed Central, https://doi.org/10.1289/ehp.10804.
García-Niño, Wylly Ramsés, and José Pedraza-Chaverrí. “Protective Effect of Curcumin against Heavy Metals-Induced Liver Damage.” Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, vol. 69, July 2014, pp. 182–201. PubMed, https://doi.org/10.1016/j.fct.2014.04.016.
Ghafouri-Fard, Soudeh, et al. “Nrf2-Related Therapeutic Effects of Curcumin in Different Disorders.” Biomolecules, vol. 12, no. 1, Jan. 2022, p. 82. PubMed Central, https://doi.org/10.3390/biom12010082.
GSTM1 Glutathione S-Transferase Mu 1 [Homo Sapiens (Human)] – Gene – NCBI. https://www.ncbi.nlm.nih.gov/gene?cmd=Retrieve&dopt=full_report&list_uids=2944. Accessed 24 May 2022.
Gundacker, Claudia, et al. “Genetic Background of Lead and Mercury Metabolism in a Group of Medical Students in Austria.” Environmental Research, vol. 109, no. 6, Aug. 2009, pp. 786–96. PubMed, https://doi.org/10.1016/j.envres.2009.05.003.
Harari, Raúl, et al. “Exposure and Toxic Effects of Elemental Mercury in Gold-Mining Activities in Ecuador.” Toxicology Letters, vol. 213, no. 1, Aug. 2012, pp. 75–82. PubMed, https://doi.org/10.1016/j.toxlet.2011.09.006.
Jadán-Piedra, Carlos, et al. “In Vitro Evaluation of Dietary Compounds to Reduce Mercury Bioavailability.” Food Chemistry, vol. 248, May 2018, pp. 353–59. PubMed, https://doi.org/10.1016/j.foodchem.2017.12.012.
Joshi, Deepmala, et al. “Therapeutic Potential of N-Acetyl Cysteine with Antioxidants (Zn and Se) Supplementation against Dimethylmercury Toxicity in Male Albino Rats.” Experimental and Toxicologic Pathology: Official Journal of the Gesellschaft Fur Toxikologische Pathologie, vol. 64, no. 1–2, Jan. 2012, pp. 103–08. PubMed, https://doi.org/10.1016/j.etp.2010.07.001.
Mohammad Abu-Taweel, Gasem, and Zarraq Al-Fifi. “Protective Effects of Curcumin towards Anxiety and Depression-like Behaviors Induced Mercury Chloride.” Saudi Journal of Biological Sciences, vol. 28, no. 1, Jan. 2021, pp. 125–34. PubMed, https://doi.org/10.1016/j.sjbs.2020.09.011.
Nebert, Daniel W., and Vasilis Vasiliou. “Analysis of the Glutathione S-Transferase (GST) Gene Family.” Human Genomics, vol. 1, no. 6, Nov. 2004, pp. 460–64. PubMed Central, https://doi.org/10.1186/1479-7364-1-6-460.
Oliveira, Vitor Antunes, et al. “Mercury Toxicity in Pregnant and Lactating Rats: Zinc and N-Acetylcysteine as Alternative of Prevention.” Environmental Science and Pollution Research International, vol. 27, no. 32, Nov. 2020, pp. 40563–72. PubMed, https://doi.org/10.1007/s11356-020-09836-4.
Rice, Kevin M., et al. “Environmental Mercury and Its Toxic Effects.” Journal of Preventive Medicine and Public Health, vol. 47, no. 2, Mar. 2014, pp. 74–83. PubMed Central, https://doi.org/10.3961/jpmph.2014.47.2.74.
Rodrigues, Keuri Eleutério, et al. “Aqueous Coriandrum Sativum L. Extract Promotes Neuroprotection against Motor Changes and Oxidative Damage in Rat Progeny after Maternal Exposure to Methylmercury.” Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, vol. 133, Nov. 2019, p. 110755. PubMed, https://doi.org/10.1016/j.fct.2019.110755.
Shahcheraghi, Seyed Hossein, et al. “Nrf2 Regulation by Curcumin: Molecular Aspects for Therapeutic Prospects.” Molecules, vol. 27, no. 1, Dec. 2021, p. 167. PubMed Central, https://doi.org/10.3390/molecules27010167.
SHIRKHANLOO, Hamid, et al. “Occupational Exposure to Mercury: Air Exposure Assessment and Biological Monitoring Based on Dispersive Ionic Liquid-Liquid Microextraction.” Iranian Journal of Public Health, vol. 43, no. 6, June 2014, pp. 793–99. PubMed Central, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4475598/.
Spiller, Henry A. “Rethinking Mercury: The Role of Selenium in the Pathophysiology of Mercury Toxicity.” Clinical Toxicology (Philadelphia, Pa.), vol. 56, no. 5, May 2018, pp. 313–26. PubMed, https://doi.org/10.1080/15563650.2017.1400555.
—. “Rethinking Mercury: The Role of Selenium in the Pathophysiology of Mercury Toxicity.” Clinical Toxicology (Philadelphia, Pa.), vol. 56, no. 5, May 2018, pp. 313–26. PubMed, https://doi.org/10.1080/15563650.2017.1400555.
Trinder, M., et al. “Probiotic Lactobacilli: A Potential Prophylactic Treatment for Reducing Pesticide Absorption in Humans and Wildlife.” Beneficial Microbes, vol. 6, no. 6, 2015, pp. 841–47. PubMed, https://doi.org/10.3920/BM2015.0022.
Woods, James S., et al. “MODIFICATION OF NEUROBEHAVIORAL EFFCTS OF MERCURY BY A GENETIC POLYMORPHISM OF COPROPORPHYRINOGEN OXIDASE IN CHILDREN.” Neurotoxicology and Teratology, vol. 34, no. 5, Sept. 2012, pp. 513–21. PubMed Central, https://doi.org/10.1016/j.ntt.2012.06.004.
Zalups, R. K., and D. W. Barfuss. “Participation of Mercuric Conjugates of Cysteine, Homocysteine, and N-Acetylcysteine in Mechanisms Involved in the Renal Tubular Uptake of Inorganic Mercury.” Journal of the American Society of Nephrology: JASN, vol. 9, no. 4, Apr. 1998, pp. 551–61. PubMed, https://doi.org/10.1681/ASN.V94551.
Zhuge, Xiang-Lin, et al. “Biochemical Functions of Glutathione S-Transferase Family of Salix Babylonica.” Frontiers in Plant Science, vol. 11, 2020. Frontiers, https://www.frontiersin.org/article/10.3389/fpls.2020.00364.
https://academic.oup.com/HTTPHandlers/Sigma/LoginHandler.ashx?error=login_required&state=2d2c2864-a89b-4212-8d69-724102f0751bredirecturl%3Dhttpszazjzjacademiczwoupzwcomzjajcnzjarticlezj87zj3zj753zj4633336. Accessed 24 May 2022.