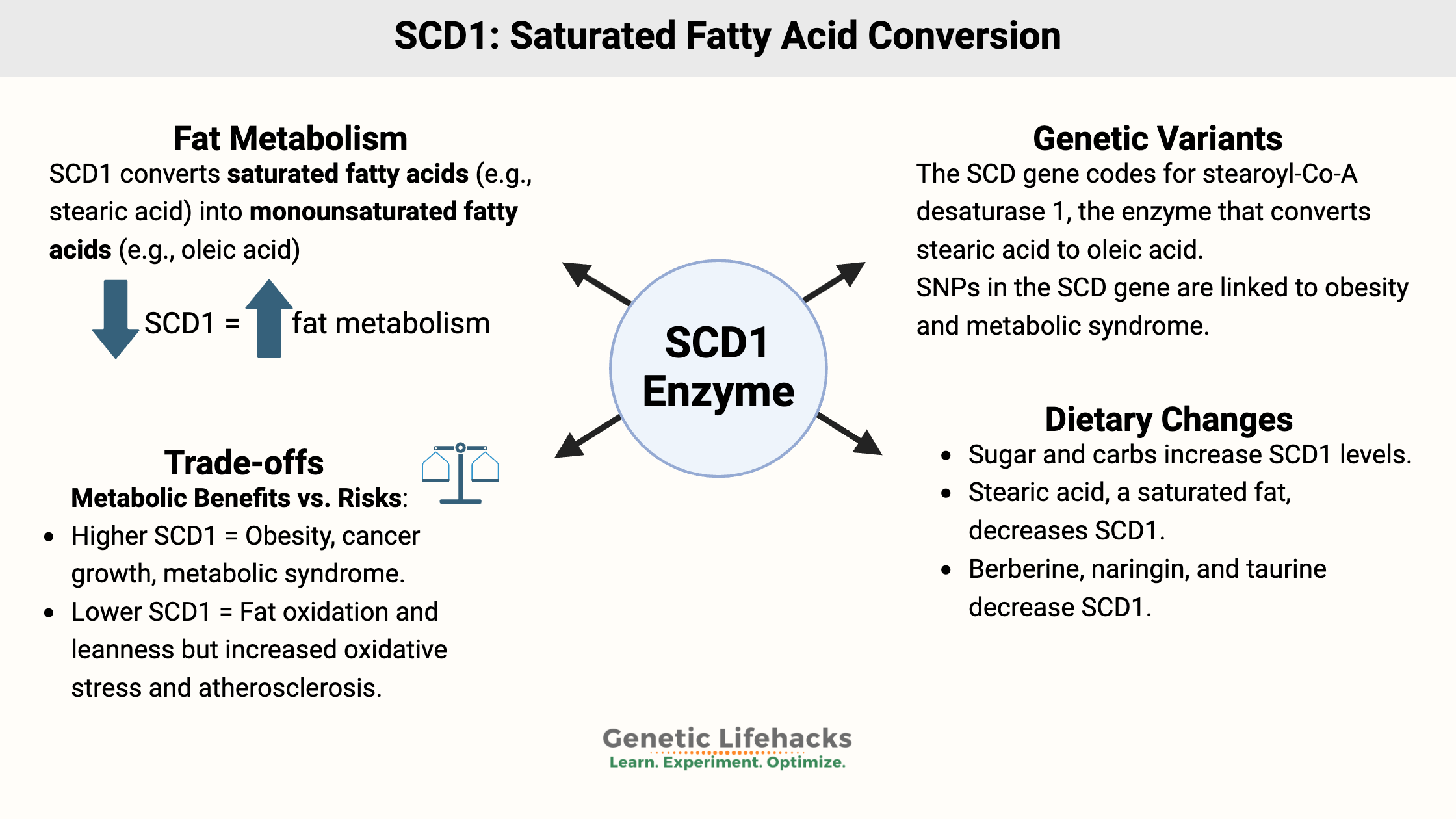
Key takeaways:
~ Many aspects of health involve the balance of utilizing fatty acids for energy, normalizing blood glucose levels, and maintaining a healthy muscle mass and weight.
~ The SCD1 gene, which encodes an enzyme central to fatty acid conversion, impacts all of these topics.
~ Genetic variants in SCD1 are linked to metabolic syndrome and weight in response to the type of dietary fat.
Stearoyl-CoA Desaturase 1 (SCD1): converting fatty acids in your cells
Stearoyl-CoA desaturase (SCD1 gene) is the rate-limiting enzyme needed for the body’s creation of monounsaturated fatty acids from saturated fatty acids.
First, some background information for context (skip ahead if you know this), and then I’ll get into the SCD1 gene and how it affects overall health, weight, and metabolic health.
Background: how your body uses food as fuel
Your cells create energy from two fuel sources: carbohydrates, which break down into glucose, and fat, which can be utilized in fatty acid oxidation for energy. When glucose is available, it is used preferentially for creating energy, and when glucose levels fall, fatty acids get used for energy.
We survive as a species because we are flexible in our ability to survive eating different diets — from hunter-gatherers eating mammoths and tubers to agrarian cultures eating mainly grains to my Irish ancestors who likely made it through famine times eating potatoes.

The body can convert glucose into fat for storage(called de novo lipogenesis), and it can convert fat back into glucose if needed (gluconeogenesis). While most cells can run on fatty acids for fuel, the brain needs glucose and thus the conversion of fat to glucose if needed.
When you eat foods containing fat, the fat gets broken down in a couple of ways with different lipase enzymes. These lipase enzymes (gastric, pancreatic) break up the fat into fatty acids and glycerol, and then bile acids act as emulsifiers, allowing the fatty acid to cross the mucous layer for absorption. (more thorough explanation here)
Background: Fatty acids in the body
While we often just think of ‘fat’ as the extra padding around our waist and hips, fatty acids have three different roles in the body:
- Fatty acids, mainly unsaturated fatty acids, make up the cell membranes of all the cells in the body.
- Fatty acids can break down for the mitochondria to use them to create energy in the form of ATP. Fatty acids are also the main way of storing fuel for later use.
- Fatty acids can act as signaling molecules, causing many different downstream effects.
So keep in mind that as we dig into this topic of saturated and monounsaturated fatty acids, the body is regulating and using these fats in multiple ways.
Background: Types of fatty acids
Fatty acids consist of carbon and hydrogen and are referred to as saturated or unsaturated. Saturated fats have all of their carbons bound to hydrogens, and unsaturated fats have at least one carbon bound to another carbon instead of a hydrogen. This change in the structure of the fatty acid differentiates saturated from unsaturated fats.
Saturated fats are generally solids at room temperature, and unsaturated fats are liquids.
Fatty acids are named based on the number of carbons in the chain and how many unsaturated double bonds between carbons exist. Saturated fats have no unsaturated double bonds. Monounsaturated fatty acids (MUFA) have one unsaturated double bond, and polyunsaturated fatty acids (PUFA) have two unsaturated double bonds.
Saturated fatty acids (# carbon atoms):
|
Unsaturated fatty acids (# carbon atoms, # unsaturated double bonds):
|
SCD1: converting saturated fat to monounsaturated fat
Getting back on topic here, the stearoyl-CoA desaturase enzyme is found in all cell types and in all animals and plants. The SCD1 gene codes for the stearoyl-CoA desaturase enzyme, which converts saturated fatty acids into monounsaturated fatty acids.
The cell membrane surrounding all of your cells consists of fatty acids. Some of those fatty acids can be saturated, but most of them need to be unsaturated, in order to give more fluidity to the cell membrane. Saturated fats, which are solids at room temperature, are less fluid in cell walls.
The stearoyl-CoA desaturase enzyme changes the bond in the saturated fat to make it unsaturated and more flexible.
Specifically, the SCD enzyme converts stearic acid (saturated fat with 18 carbons) into oleic acid (unsaturated fat, 18 carbons, and one unsaturated double bond). It can also convert palmitic acid into palmitoleic acid.
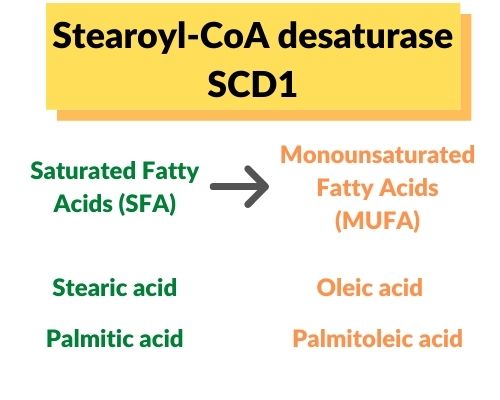
Saturated and unsaturated fats are also found in foods that you eat. Stearic acid is found in beef fat (tallow), chocolate (cacao butter), and dairy fat. Palmitic acid is found in palm oil.
I’ll come back to the dietary aspects of SCD1 in the Lifehacks section…
Does SCD1 alter your ability to burn off stored fat?
To lose weight, you have to use your stored fat for energy at a rate that is higher than your rate of replacing that fat.
Researchers discovered that SCD1 (stearoyl-CoA desaturase) is increased in people with obesity and metabolic syndrome. Additionally, people who are obese have higher ratios of monounsaturated fat to saturated fat (MUFA:SFA ratio).[ref][ref]
The question, though, is whether the increased SCD1 caused obesity or whether it was elevated in response to having metabolic syndrome.
Animal research may give us the answer. Research consistently shows that lower SCD1 levels keep the animal lean, even on a diet that normally would make them fat. The researchers decreased SCD1 levels by manipulating the gene in various tissues of the animal, seeing the results of knocking out the gene in different organs.
Why does a lower SCD1 level keep mice lean? The lack of the SCD1 enzyme causes an increase in the rate of using up fat for fuel in the mitochondria. This increased fatty acid β-oxidation is through activation of the AMPK pathway.[ref]
decreased SCD1 = increased burning of fat
Additionally, mice with SCD1 deficiency had better insulin sensitivity and reduced liver fat. They were ‘hypermetabolic’, burning off fat at a faster rate, irrespective of a fattening diet. The researchers were able to pinpoint that the deletion of SCD1 in skin and muscle tissue is what keeps the mice lean through increasing overall metabolism.[ref][ref]
While decreased SCD1 gives the benefit of decreasing weight gain, and it also increases oxidative stress in the mitochondria and stress in the endoplasmic reticulum. Specifically, it seems that the decrease in oleic acid drives the ER stress, while low palmitoleic acid does not seem to matter.[ref]
Let’s dig into some more details here…
Liberating fat from your adipose tissue:
Lipolysis is the release of fatty acids from adipose tissue for use in the body as energy or in other ways.
Most cells in the body get replaced at the end of their lifecycle. This happens quickly for some cell types, like intestinal cells, that turn over in a matter of days, and more slowly for other types, like heart cells, which turn over very slowly, if at all.
Adipose cells are around for about 9 years, on average. Interestingly, fat stored in adipose tissue has an average age of 1.4 years. This means that once the fat is stored, it doesn’t get used up and is replaced very fast. As people who are obese get older, the usage of fat from fat cells for fuel tends to slow down, and accumulation speeds up a bit.[ref]
The SCD1 enzyme is key in the fatty acid composition in your adipose tissue. Animal studies show that knocking out the SCD1 gene alters the fatty acid makeup of adipocytes.[ref]
Brown fat, beige fat, and white fat:
Brown fat, found in small amounts in adults, is an active type of fat cell that burns through a lot of fuel and produces a lot of heat. Lean people usually have more brown adipose tissue, and people who are obese usually have little to no brown fat. Brown fat is brown due to the high number of mitochondria in each cell, and it helps to both produce heat and burn off excess energy.[ref]
White fat (white adipose tissue) is what you typically think of as fat cells. It is a storage cell, keeping fat on board for a time of famine. Not as many mitochondria, not producing the heat or energy that brown fat does.
Recently, researchers discovered that white adipose tissue can be induced to become more like brown adipose tissue. This in-between fat cell type is termed beige or brite adipose tissue. Exposure to cold is one way that white adipose tissue can be induced to become the heat-producing, metabolically active beige fat.[ref]
A 2020 research paper published in PNAS shows that when adipose cells are formed from stem cells, they can take two paths: white or beige. SCD1 is key to which path the stem cells take. The animal study showed that decreased SCD1 caused the stem cells to differentiate into beige adipose tissue rather than white. The decreased SCD1 only affected the formation of new adipose cells – it didn’t convert existing white adipose tissue to beige.[ref]
Succinate is an intermediary formed when the mitochondria produce ATP in the electron transport chain. The researchers also found that the rising succinate levels due to the lack of SCD1 were the trigger for beige adipose differentiation. In cell culture, succinate alone is capable of causing adipose stem cells to differentiate as beige cells instead of white. The increase in succinate accompanies an increase in reactive oxygen species (ROS) in the cell.
To bring this full circle, the researchers then examined how the fatty acid composition changed in the adipose stem cells due to SCD1 deficiency. The lack of SCD1 caused an increase in the ratio of saturated fat to monounsaturated fat. Adding oleic acid, a monounsaturated fat, to cell cultures lacking SCD1 caused succinate levels to drop back to normal. The whole study is available open access and worth reading if you want to get geeky.
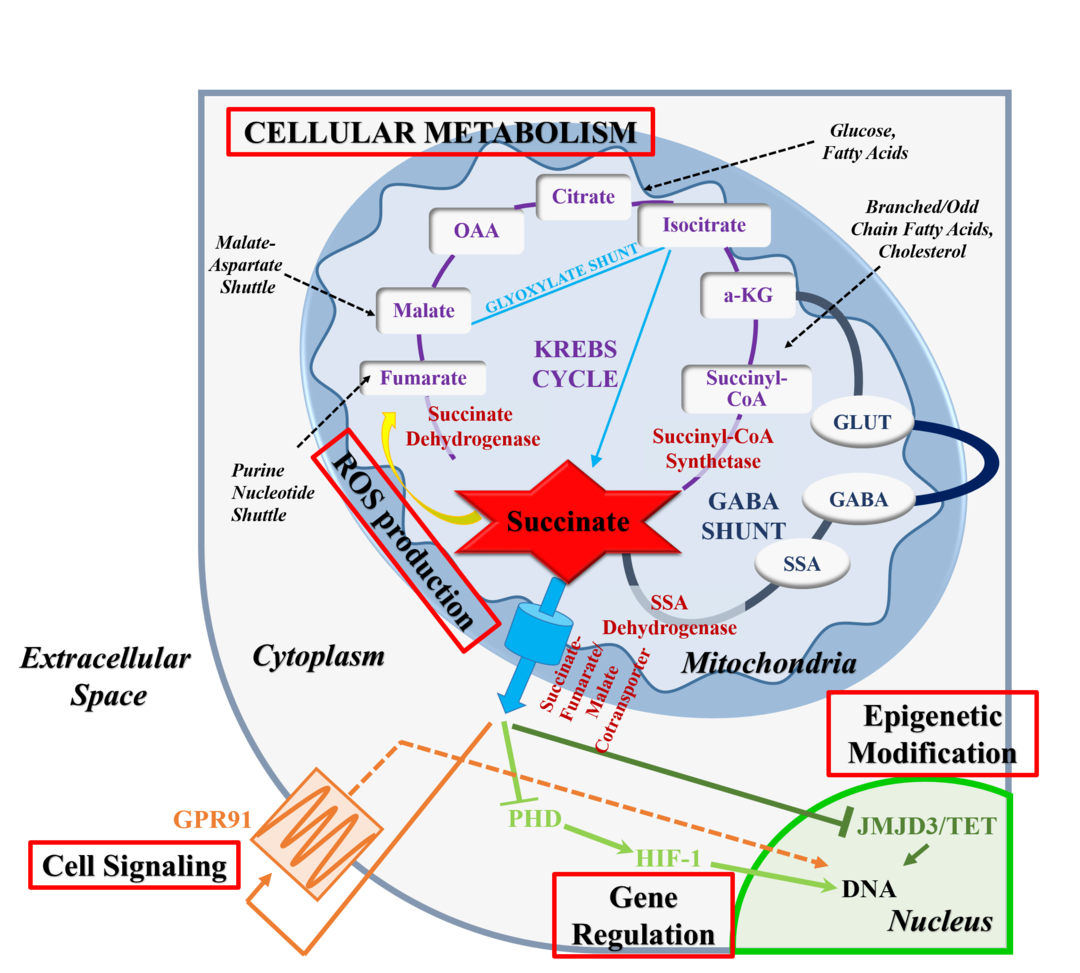
Mitochondrial activity in white adipose tissue:
While studies on brown fat and the beige-ing of white fat in animals are interesting, a new study shows that white adipose tissue may be the deciding factor in keeping people naturally lean. The study looked at people with ‘constitutional thinness’ defined by a BMI under 18 kg/m2 and maintaining long-term while eating a normal number of calories.
The results showed the mitochondria in white adipose tissue of lean people were both more active and more numerous. To get even more specific, mitochondrial activity at complex II was higher in people who were constitutionally thin.[ref]
What does this have to do with SCD1? Well, stick with me here for a minute.
Stearic acid uses SCD1. What happens when you feed people stearic acid? Their white adipose tissue mitochondria use more energy from fat and tend to fuse together.[ref]
While we often imagine mitochondria as little jellybean-shaped ‘powerhouses of the cell’, they are actually flexible and changeable organelles. Mitochondria can divide (fission), fuse together, and travel between cells. Mitochondrial fusion allows mitochondria in metabolically active cells to share resources, absorb damaged mitochondria, and produce more energy.[ref]
Menopause and weight gain:
A 2019 animal study found that SCD1 methylation levels are inversely correlated with menopausal age. Methylation is one way that the body can ‘turn off’ a gene so that it isn’t translated into the enzyme. So as methylation of SCD1 decreases (the turning off gets taken away), there is more SCD1 produced as the years go by after menopause.[ref]
This increase in SCD1 via epigenetic regulation may be one cause of weight gain after menopause.
Beyond weight – SCD1 impacts other functions also
SCD1 in myelination and neurodegenerative diseases
Phagocytes, a type of immune cell that engulfs and removes damaged cells, increase in diseases affecting neurons, such as multiple sclerosis. Newer research points to the importance of phagocytes for removing damaged myelin for repair.
A new study points to SCD1 playing a key role in this process. Higher levels of SCD1 cause an increase in monounsaturated fatty acids, which, in the case of demyelination diseases such as MS, cause a damaging foam cell to form. These foamy phagocytes are unable to clear out the damaged myelin, which prevents the neurons from being able to regenerate.[ref]
Decreasing SCD1 then allows for the phagocytes to remove the damaged myelin sheath. This may be one mechanism through which lower carbohydrate diets that are high in meat (saturated fats), such as the Wahls protocol, seem to help people with MS.
A study involving the brains of Alzheimer’s patients determined that their MUFA to Saturated Fatty Acid (SFA) ratio was higher than in normal brains. Unsurprisingly, they found higher SCD1 levels in Alzheimer’s brains. Additionally, people with higher MUFA to SFA ratios also had lower scores on memory tests.[ref]
SCD1 in cancer:
Cancer cells multiply quickly and have a unique metabolism. Studies show the increase of SCD1 happens in many types of tumor cells and facilitates cancer growth.[ref][ref]
Keep in mind also that people who are obese usually have higher than average SCD1 levels. Perhaps this is one reason morbid obesity (BMI>40) increases the risk of several types of cancer by 1.5-fold.[ref]
In colon cancer, higher SCD1 levels combined with (caused by?) a high-carb diet cause an increase in metastasis and poor prognosis. Increasing MUFA in the diet and decreasing carbs may reverse this situation.[ref]
SCD1 in non-alcoholic fatty liver disease
Levels of SCD1 are increased in non-alcoholic fatty liver disease (NAFLD), but only when caused by excess fat consumption.[ref]
SCD1 in viral replication:
About 170 million people worldwide have hepatitis C, which is an enveloped RNA virus. Hepatitis C ends up killing about 350,000 people a year through liver failure. SCD1 turns out to play a key role in the proliferation of the virus. As an enveloped virus, hepatitis C uses SCD1 in the formation of the viral membrane. Inhibiting SCD1 reduces the replication of the virus.[ref]
SCD1 is also vital to the replication of other enveloped positive-strand RNA viruses – dengue virus and all flaviviruses. Essentially, the virus needs the flexibility of MUFA in the formation of the membrane, and without SCD1, it can’t replicate.[ref][ref]
Tradeoffs: If increased SCD1 is so bad, why does the body keep doing it?
It seems that everything so far points to higher SCD1 levels being ‘bad’ — increased obesity, cancer metabolism, neurodegeneration, etc.
You may be wondering if we could all just take an SCD1 inhibitor pill and all be lean and healthy.
There is always a flip-side, a reason the body does something.
SCD1 in atherosclerosis
Animal studies consistently point to a downside to decreasing SCD1: increased atherosclerosis.[ref]
Heart disease is the number one killer worldwide, so promoting atherosclerosis (plaque in the arteries) is not good…
Why would low SCD1 increase atherosclerosis? This seems to be a question that researchers are still trying to figure out. One thing the research shows is that SCD1 deficiency changes cholesterol homeostasis and also raises levels of IL-6, an inflammatory cytokine.[ref][ref]
An interesting mouse study, though, gives one possible solution to this conundrum. In it, the researchers inhibited SCD1, which predictably prevented metabolic syndrome, but they also gave the animals fish oil. This mitigated the impact of atherosclerosis.[ref]
SCD1 is needed in humoral immunity:
The SCD1 production of MUFA is essential for the creation of B-cells, part of the body’s immune system. A recent study from the Mayo Clinic shows that you need enough SCD1 available for humoral immunity and for the immune response to vaccines. The study (preprint) shows the SCD1 creation of monounsaturated fatty acids in B-cells is needed for the maturation of the cells and, thus, for a good immune response to a pathogen. The study specifically focused on influenza A and the response to both infection and immunization.[ref]
Insulin Resistance and SCD1: trade-offs and context
The production of insulin, a hormone, occurs in the pancreas in response to blood glucose levels. Insulin docks with insulin receptors on the cell membrane, signaling for a cascade of events inside the cell, which results in the cell taking in glucose.
A component of metabolic syndrome or diabetes is insulin resistance (IR), which is when cells aren’t responsive to the insulin signal. This results in higher glucose levels in the bloodstream.
SCD1 plays an important role in insulin regulation through fatty acid levels. Leptin, a hormone that regulates appetite due to lipid levels, acts upon SCD1.[ref]
A large Mendelian Randomization study showed that insulin resistance is causally related to lower levels of polyunsaturated fatty acids, including oleic acid and palmitoleic acid.[ref] Essentially, Mendelian randomization studies take what is known about genetic variants that cause a change in a biomarker and apply that information to determine causality — for example, does low oleic acid cause insulin resistance, or does insulin resistance cause low oleic acid. In this case, the genetic variants that cause insulin resistance also causes lower oleic and palmitoleic acid.
SCD1 increases oleic and palmitoleic acid levels and is also linked to weight gain. Downregulating SCD1 causes insulin resistance but also decreases obesity. The flip side is that it may increase atherosclerosis in some people through the increase in saturated fatty acids in LDL cholesterol. (Read the whole study here)
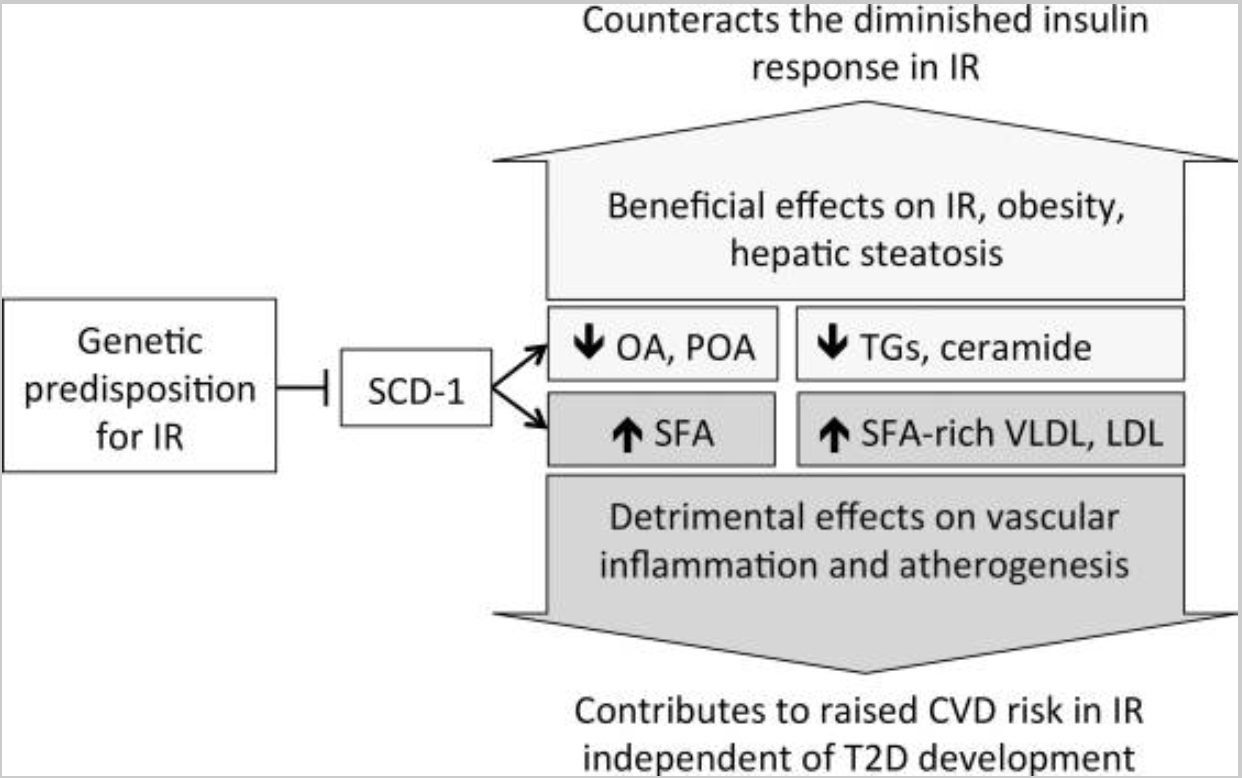
Is causing insulin resistance a bad thing? It depends on your diet and lifestyle.
The combination of insulin resistance along with decreased insulin production causes type 2 diabetes. Thus, in the context of a diet causing higher blood glucose levels (e.g., high in sugar, and carbs), insulin resistance combined with decreased insulin production is not good, to say the least.
A ketogenic diet (high fat, low carb) also causes transient insulin resistance, which reverses upon resumption of a diet that contains carbs.[ref] So, in the context of a diet that is low carb and doesn’t trigger the release of insulin, temporary insulin resistance may not be detrimental.
SCD1 Genotype Report
Lifehacks for decreasing SCD1 for weight loss:
The idea of reducing SCD1 levels to increase overall metabolism and cause weight loss is tantalizing.
Keep in mind the trade-offs with inhibiting SCD1 — mainly decreased immune response and atherosclerosis. Everyone is unique in their risk factors for atherosclerosis, but do keep it in mind if you are making any long-term dietary changes.
Everyone is also unique in their best bet for weight loss. Intriguing to me is the strong connection between the FTO variant, carbohydrate intake, and SCD1 gene expression.[ref]
I also want to caution that a lot of the studies on stearoyl-CoA desaturase are done in animals (usually mice). While it is interesting to see the results of these studies, I always keep a little kernel of doubt in the back of my mind. Example: The SCD1 studies show that decreased enzyme function in skin and muscles decreased weight… This could be due to the mice being cold (less fat under the skin), causing an increase in brown fat.[ref]
Dietary changes that decrease SCD1:
Related Articles and Topics: