Key takeaways:
~ Statins inhibit HMGCR, which is the rate-limiting enzyme of the mevalonate pathway.
~ The mevalonate pathway produces cholesterol, CoQ10, and other compounds, and statins decrease the production of these compounds.
~ Some studies show that statins may cause memory problems and brain fog. Both cholesterol and CoQ10 are important in cognitive function.
~ Genetic variants impact how well statins inhibit the HMGCR enzyme in the mevalonate pathway.
This article explores the research on the complex relationship between statin use and brain fog, exploring how these cholesterol-lowering medications might influence cognitive function.
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Consider joining today.
How and why statins may cause cognitive problems – in some people
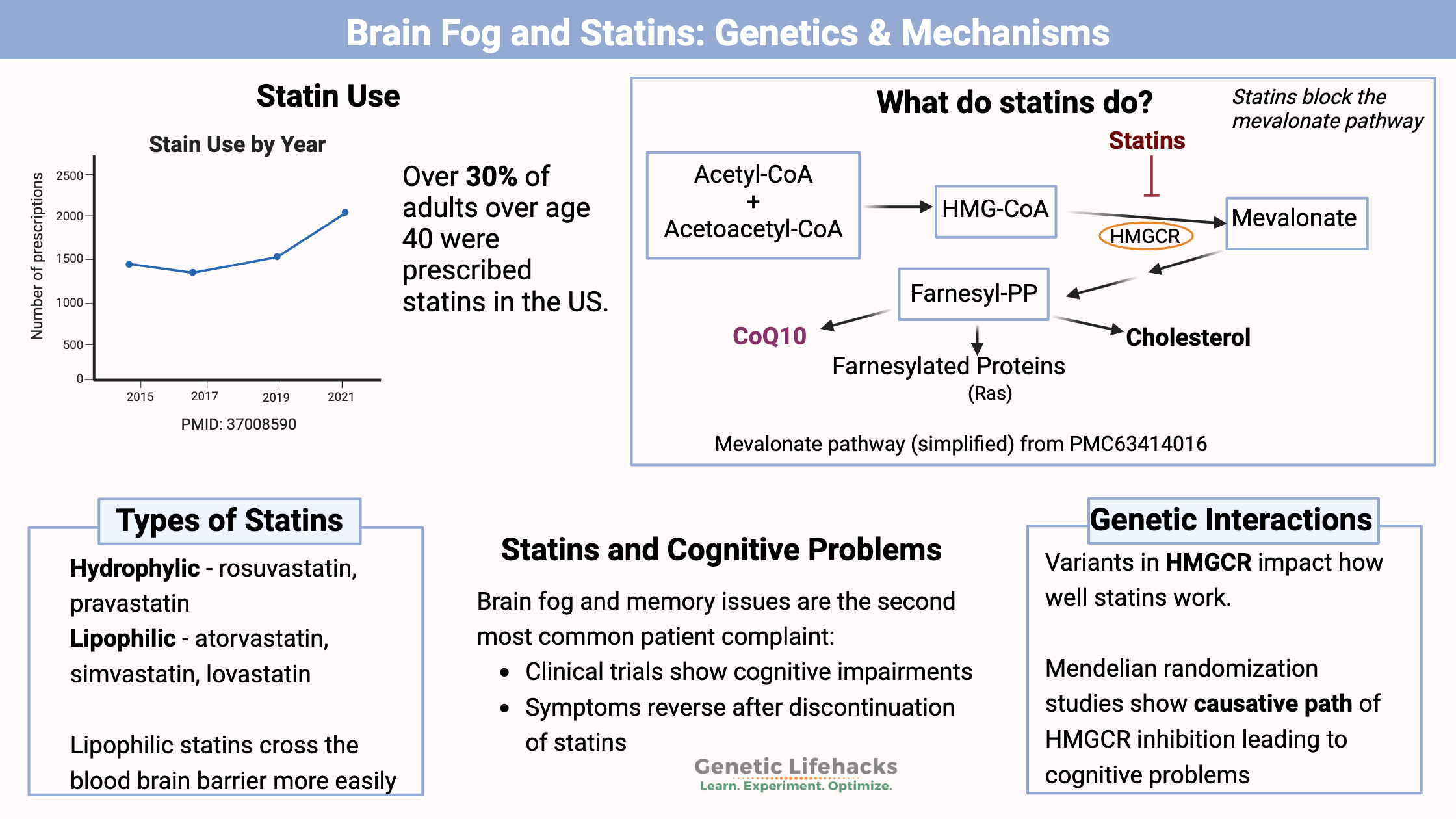
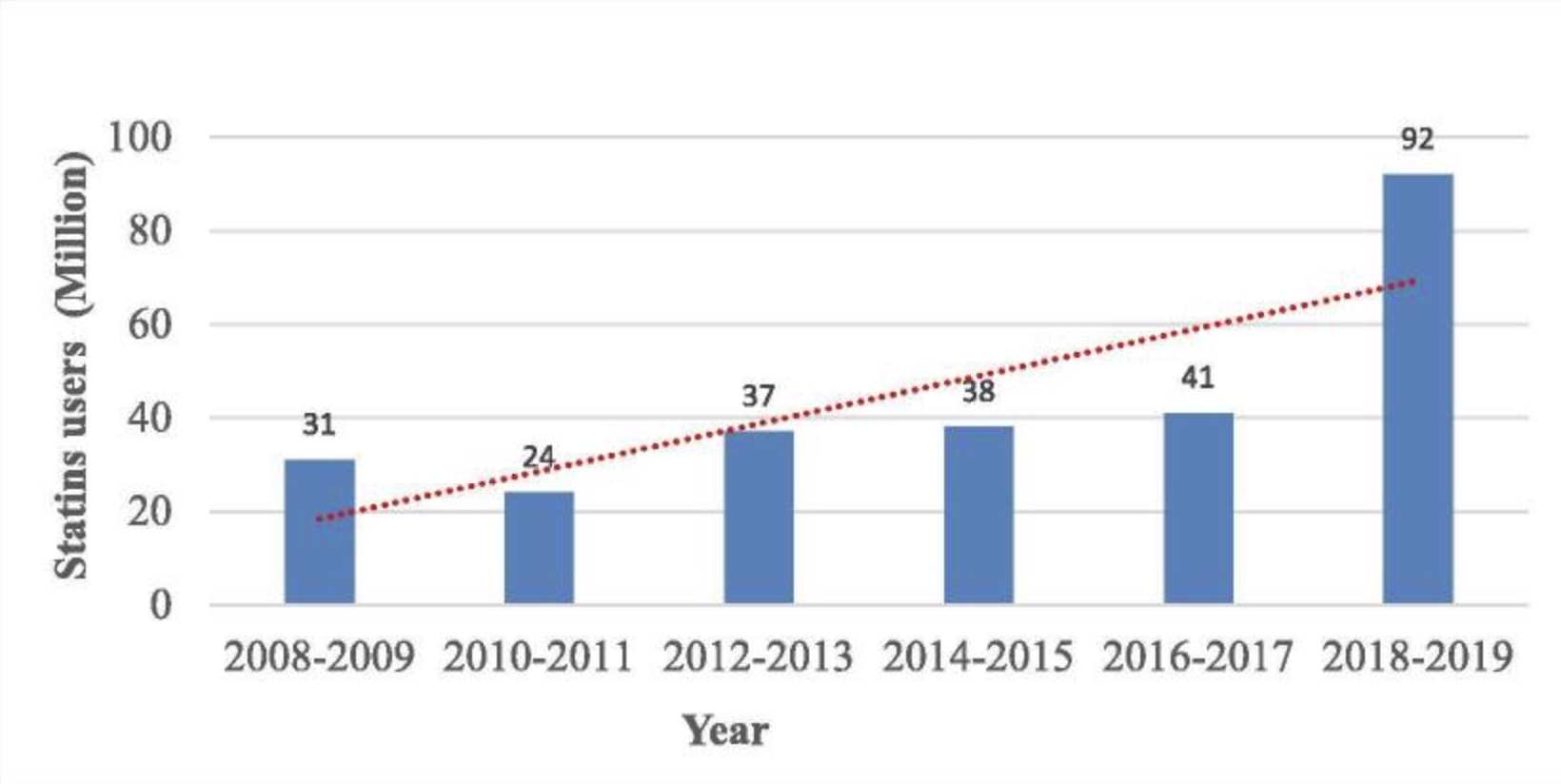
Statins are a type of cholesterol-lowering medication. They have been prescribed for decades in people with very high cholesterol, and now they are recommended to people with any risk for heart disease. Currently, about 92 million people in the US are on a statin.[ref]
However, there is more to the story of statins than just heart disease…
The second most common complaint of statin users is brain fog or memory issues. This article examines how statins, commonly used for managing cholesterol, might contribute to cognitive issues such as brain fog.
Over 30% of adults over age 40 in the US are on a statin, and one of the most common patient complaints is a “cognitive impairing effect in some patients”, according to a study in 2018.[ref]
While statins used to be prescribed just for high LDL cholesterol, new guidelines from the American Heart Association recommend statins for all patients between the ages of 40 and 75 who are at increased risk of heart disease, regardless of their LDL-C levels.[ref] In the US, statins are now a $10 billion per year drug class.[ref] A new study (July 2024) lays out how the pharmaceutical industry uses “nudges” to increase statin use by targeting primary care physicians who hadn’t prescribed statins based on electronic health records.[ref]
Cholesterol Synthesis: Statins and the Mevalonate Pathway
I’ll start with how cholesterol is made and how statins work to decrease cholesterol synthesis — and then go into the connection with CoQ10, brain fog, and memory issues.
Cholesterol that circulates in the bloodstream can come from the foods you eat, or it can be synthesized in the liver. Absorption of cholesterol from food is poor, so most cholesterol is synthesized in liver cells. All of your other cells can also make cholesterol in smaller amounts.[ref]
Cholesterol synthesis in cells starts with acetyl-CoA, which is then converted to mevalonate.
This is where statins come in…
Statins block the mevalonate pathway, which reduces the production of cholesterol (and other substances, such as CoQ10).
How do statins reduce cholesterol?
Statins are a fungal metabolite that inhibits the mevalonate pathway, the pathway that cells use to make cholesterol. Here’s a diagram that explains the mevalonate pathway:
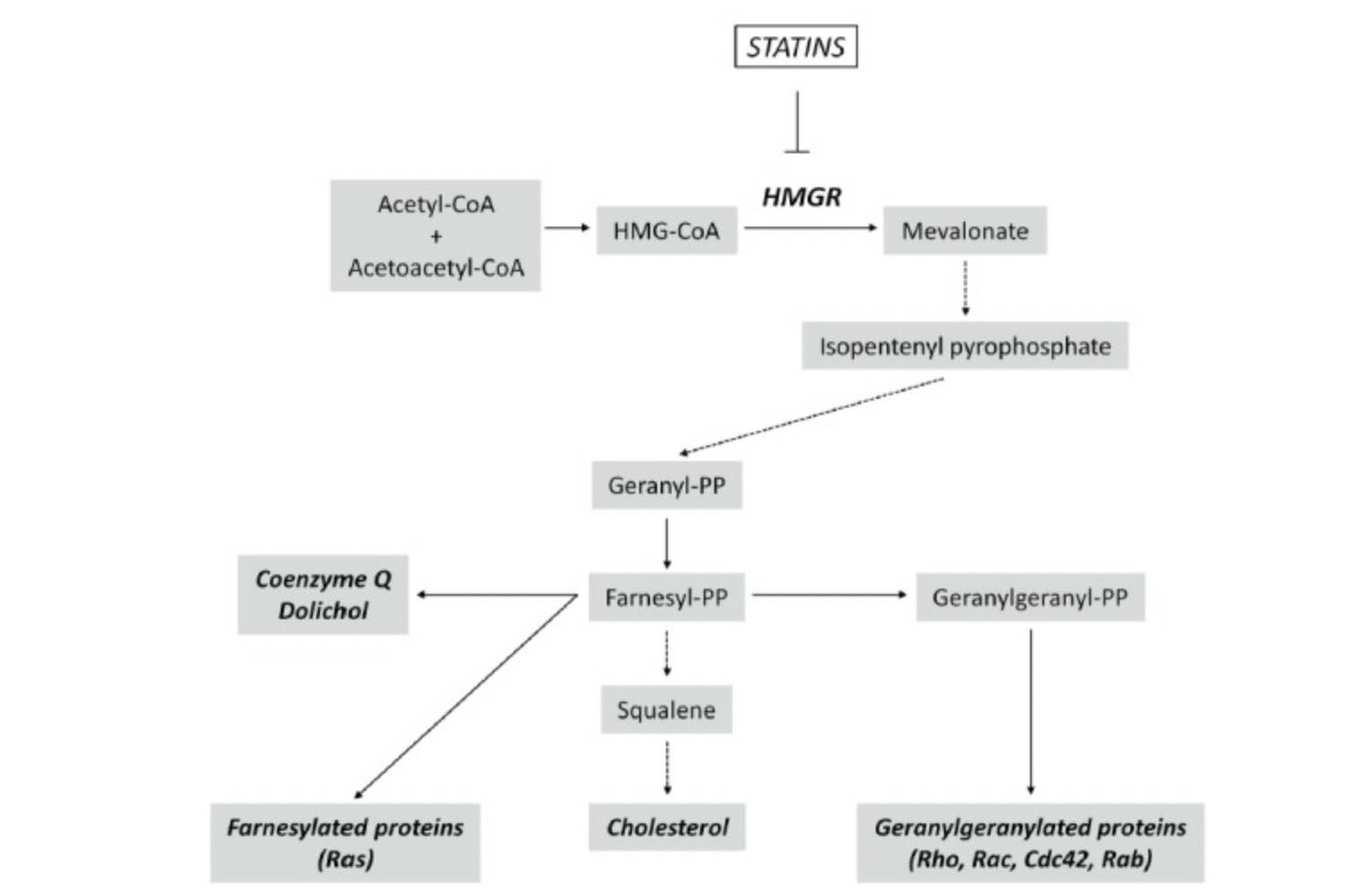
Statins block the mevalonate pathway by inhibiting the enzyme HMG-CoA reductase (HMGCR).
The mevalonate pathway is the way cells produce the building blocks needed to synthesize cholesterol, coenzyme Q, and other proteins.
Inhibition of the HMGCR enzyme causes a significant reduction in cholesterol production, resulting in increased extracellular uptake of plasma LDL- LDL cholesterol (LDL-C). This results in lower circulating LDL-C levels.[ref]
Before we get into statin-induced memory issues, let’s look at what cholesterol does in the body.
| Pathway/Compound | Statin Effect | Relevance to Brain Fog/Cognition |
|---|---|---|
| Cholesterol Synthesis | Decreases | Essential for myelin, synapses |
| CoQ10 Production | Decreases | Needed for mitochondrial energy |
| Blood Glucose | Increases risk of diabetes | Diabetes linked to cognitive decline |
What does cholesterol do in the body?
Cholesterol is incorporated into the cell membrane of all cells. Within the cell membrane, cholesterol makes the membrane more stable and decreases fluidity. This is an essential role of cholesterol in all animals, and cholesterol makes up about 30% of animal cell membranes.
Cholesterol is also used in the synthesis of steroid hormones (progesterone, testosterone, estrogen, cortisol, aldosterone, etc.), as well as in the biosynthesis of bile acids and vitamin D.
Cholesterol is completely essential — in the right amount. A very high level of cholesterol due to rare genetic mutations, though, is detrimental.
Familial hypercholesterolemia is high cholesterol (LDL-C >190 mg/dL) caused by mutations in the LDL receptor, APO-B, or PCSK9, which drive cholesterol production in the liver. It is not caused by dietary cholesterol.[ref][ref]
Related article: LDL cholesterol genes
Statins were developed to treat familial hypercholesterolemia. They inhibit HMGCR and reduce cholesterol production in the liver of people with hypercholesterolemia.
Cholesterol in Brain Health:
The brain contains approximately 25% of the body’s cholesterol. The blood-brain barrier doesn’t allow circulating cholesterol to pass through easily, so de novo synthesis by brain cells is the source of almost all cholesterol.[ref]
Thus, brain cholesterol is entirely dependent on the mevalonate pathway.
The right amount of cholesterol is essential for brain function:
- The myelin sheath is a layer that acts like insulation around the axons of nerve cells in the brain. It is composed of cholesterol and other fatty acids. The myelin sheath is a critical part of how nerve cells in the brain function, and damage to the myelin sheath is associated with neurological diseases, such as MS. [ref]
- Cholesterol is also essential in the synapses of neurons – the areas responsible for transmitting the signal to the next neuron. The synapse releases neurotransmitters in small vesicles, and cholesterol is important in making up the vesicles that surround the neurotransmitters.[ref]
Decreasing cholesterol in the brain can impact cognitive function, but this is only half of the picture…
The mevalonate pathway in the brain: CoQ10 impacted
Statins partially block the mevalonate pathway, reducing the production of cholesterol, coenzyme Q, and prenylated proteins.
Lipophilic statins can cross the blood-brain barrier and inhibit the mevalonate pathway in neurons.[ref] Some epidemiologic studies suggested a possible benefit of statins in terms of Alzheimer’s risk, but randomized controlled trials have failed to show a benefit.[ref] Reducing cholesterol in the brain can have both positive and negative effects, depending on how much cholesterol production is inhibited and whether cholesterol production was normal to begin with.
The use of cholesterol in the vesicles that release neurotransmitters means that optimal levels of cholesterol are necessary for brain function. Studies show that too much – or too little – cholesterol reduces the production of GABA, an essential inhibitory neurotransmitter.[ref][ref]
Related article: GABA
In addition, research shows that reducing cholesterol levels in the brain can lead to the loss of neuronal synapses.[ref]
Statins also reduce the production of coenzyme Q (CoQ10), which is essential for energy production in the mitochondria. CoQ is used in mitochondria for electron transport during oxidative phosphorylation. It also acts as an antioxidant in neurons, protecting against neuronal damage.[ref]
Related article: CoQ10 genes, mitochondrial energy, and supplement research
Mitochondria produce ATP, the energy needed for cellular functions. Within the mitochondria, there is an electrochemical gradient that moves protons, resulting in stored energy in ATP. Part of this electron transport system involves coenzyme Q (also called CoQ10 or ubiquinone). Research shows that statins decrease the production of CoQ10, which directly causes mitochondrial dysfunction.[ref]
Studies on statins and cognitive function:
Before I get into the studies on statins and memory problems, I want to explain that this is a well-known problem. The FDA added a safety label to statins in 2012 that includes cognitive adverse events such as “notable, but ill-defined memory loss or impairment that was reversible upon discontinuation of statin therapy.”[ref]
Statins were developed to reduce the risk of cardiovascular disease by lowering LDL cholesterol. As such, most clinical trials of the various statin drugs have focused on cardiovascular outcomes and have not examined cognitive side effects.
The most common side effect reported in clinical trials of statins is muscle pain, with 5-29% of patients experiencing muscle symptoms due to the effect of statins on mitochondrial function and muscle protein breakdown.[ref]
Related article: Genetic variants linked to statin muscle pain
The second most common side effect reported for statins is memory or cognitive changes.[ref][ref]
Here are some of the clinical trials that specifically looked at cognitive changes in statin users.
Decreased processing: A placebo-controlled trial published in 2000 found that the group taking lovastatin (20mg) had small but statistically significant decreases in scores for attention and processing speed after six months on lovastatin, compared with the placebo group. [ref]
Patient-reported side effects:
A study that parsed patient reviews of statins from online sources such as medications.com and askpatients.com also found that statin users quite often reported side effects related to loss of mental clarity and forgetting words.[ref]
Decreased cognitive functioning:
Another placebo-controlled study in 2004 found “minor decrements in cognitive functioning with statins”.[ref]
A review of 60 case studies found that 50% 0f the patients noticed cognitive impairment in the first two months of statin usage, and over half of the patients returned to normal when statins were discontinued.[ref]
The length of the clinical trial may be important here. Another study looked at cognitive performance for a month after statins and found no statistical difference on “computerized performance tests”.[ref]
Short-term decline in cognitive performance:
A study on statin users aged 40-69 found that statins did cause a short-term decline in cognitive performance due to lower cholesterol and higher blood glucose concentrations in the brain. However, the researchers concluded that at the end of 8 years, there is no long-term adverse effect on cognition.[ref]
Symptom reversal with stopping the drug:
The good news is that the effects of statins on memory are usually reversible. A survey of 171 patients who had reported memory or cognitive impairment with statins showed that 90% of patients who stopped the statin had improvements in their symptoms.[ref]
Not all studies agree:
- From one meta-analysis: “Short-term trials did not show a consistent effect of statin therapy on cognitive end points.”[ref]
- A prospective Australian study of elderly adults also found that statins did not cause greater memory decline. The study involved hundreds of older adults, some of whom had been taking a statin for an average of nine years before the study began. The results showed no difference in the amount of cognitive decline between the statin group and the non-statin group.[ref] Keep in mind, though, that the statin group’s baseline was nine years after starting the statin…
A review study in 2017 sums up the data: “To date, most studies of statins and cognition have been observational, with few randomized controlled trials… However, increasing concern exists that statins may be a causative factor for cognitive problems.”[ref]
With 92 million Americans taking statins (2019 numbers[ref]), this is an important question that needs better answers, including unbiased studies that measure cognitive changes in new statin users.
Types of statins: Crossing the blood-brain barrier
So far, I’ve talked in general terms about statins as a class of HMGR inhibitors. However, there are different types of statins – with different effects.
Types of statins:
Statins can be classified as hydrophilic, dissolving in water, or lipophilic, dissolving in a lipid. Lipophilic compounds can be more easily absorbed through the cell membrane and can cross the blood-brain barrier.[ref]
Statin Types and Blood-Brain Barrier Penetration
| Statin Type | Examples | Ability to Cross Blood-Brain Barrier | Notes |
|---|---|---|---|
| Lipophilic | Atorvastatin, Simvastatin, Lovastatin | Yes | More likely to affect the brain |
| Hydrophilic | Rosuvastatin, Pravastatin | No/Minimal | Primarily acts in the liver |
In the US, the most commonly prescribed statins are atorvastatin and simvastatin, both of which are lipophilic statins.[ref]
Pravastatin is hydrophilic and doesn’t cross the blood-brain barrier very well. A large trial of pravastatin in elderly adults found no statistical decrease in cognition.[ref]
Statins and the increased risk of type 2 diabetes: Another link to brain fog?
Many studies show that statin use increases the risk of type 2 diabetes:
- A study using health data from over 500,000 patients showed that statin use for more than one year increased the risk of type 2 diabetes by 2-fold.[ref]
- Another study that followed >13,000 patients for 7 years also showed a similar increase in type 2 diabetes risk with statin use. This study broke it down by type of statin and found that multi-year use of simvastatin and atorvastatin was associated with the greatest increase in type 2 diabetes cases.[ref]
- A large study concluded: “use of atorvastatin, rosuvastatin, pitavastatin and simvastatin had significant association with increase in fasting glucose. Pravastatin, lovastatin, and fluvastatin had non-significant trend toward an increased fasting glucose.”[ref]
The CDC explains that “using statins increases blood sugar because statin use can stop your body’s insulin from doing its job properly.”[ref]
Is blocking HMGCR causal for diabetes or just coincidental?
Researchers can use genetics to figure out causality. Multiple studies show that people with genetic variants that decrease HMGCR have higher rates of insulin resistance, higher BMI, and decreased pancreatic insulin release. Animal studies show this as well.[ref]
Diabetes, both type 1 and type 2, can decrease cognitive function. For more than a hundred years, it has been noted that diabetes causes poor memory and a lack of attention. Meta-analyses of multiple studies show cognitive declines in multiple domains of cognitive function in people with diabetes.[ref]
Tradeoffs and benefits of taking statins:
I don’t want to give a one-sided picture here. There are multiple effects of statins, with positive benefits in certain situations.
Benefits of statins in preventing and treating cancer:
One benefit of statins is seen in the treatment of certain types of brain tumors. Blocking the mevalonate pathway stops proliferation and cell growth in the brain, which is actually a good thing when it comes to brain tumors. In one study, researchers estimated that simvastatin and lovastatin users were almost 50% less likely to have gliomas. (NSAID users also had a similar protection against glioma.)[ref]
Reducing HMGCR is also associated with a reduced risk of ovarian cancer.[ref]
Beyond HMGCR inhibition: Other statin benefits to take into consideration
Lovastatin is also a TLR4 antagonist. Toll-like receptor 4 is part of the immune response to viruses and bacteria, and lovastatin specifically inhibits the activation of TLR4 (but not other toll-like receptors).[ref] While it may intuitively seem like a bad idea to inhibit part of the immune system, in certain situations, it may be beneficial to reduce inflammation.
Simvastatin is also a ‘bone anabolic agent’. In animal models, simvastatin, a polyethylene glycol (PEG)-based prodrug, forms micelles that are taken up by cells in bone fractures. The study suggests that simvastatin may promote fracture healing.[ref] However, a large study of rosuvastatin showed no benefit for preventing fractures in older people.[ref]
Cholesterol reduction for Alzheimer’s?
APOE is a cholesterol-carrying molecule, with APOE E4 increasing the risk of Alzheimer’s. Thus, reducing cholesterol has been studied for its effect on Alzheimer’s patients.
- A 2025 study found that statins increased the relative risk of Alzheimer’s by 20-30% in everyone except for APOE E4/E4 carriers.[ref]
- A trial that looked specifically at APOE4 and statins found no benefit for statins plus evolocumab (monoclonal antibody).[ref]
- Another study using just evolocumab to reduce cholesterol also found no difference in cognitive decline compared to placebo.[ref]
- However, some studies show that statins may be beneficial. A study involving mostly African Americans showed that statins in combination with the APOE E4 allele were beneficial in reducing the annual decline in memory.[ref]
Related article: APOE and Alzheimer’s
Statins for reducing heart attacks and strokes: absolute risk reduction is small
The reason most people take a statin is to reduce the risk of dying from a heart attack or having a stroke. A 2022 analysis published in JAMA calculated the actual absolute risk reduction from statin use, based on data from 21 large, randomized clinical trials with 2+ year durations. The all-cause mortality absolute risk reduction provided by taking a statin was less than 1%. The absolute risk reduction for stroke was only 0.4%, while the number of heart attacks was reduced by 1.3%.[ref]
The authors of the JAMA study concluded, “The study results suggest that the absolute benefits of statins are modest, may not be strongly mediated through the degree of LDL-C reduction, and should be communicated to patients as part of informed clinical decision-making as well as to inform clinical guidelines and policy.”[ref]
Genetic studies show the link between statins and brain fog:
Mendelian randomization studies are used to determine whether associations are likely to be causal. Using this type of study, scientists take well-known genetic variants that affect a pathway and see if they also affect a phenotype.
In this case, researchers can determine whether inhibiting HMGCR causes a reduction in cognitive function and compare it to reducing cholesterol in other ways.
A Mendelian randomization study showed that targeting PCSK9 for lowering cholesterol doesn’t impair cognitive performance, memory, or brain size. This would correlate with genetic variants in the PCSK9 gene that lead to lower cholesterol over a lifetime. However, the analysis showed that inhibiting the mevalonate pathway had starkly different results. HMGCR inhibition, which is what statins do, does reduce cognitive performance, reaction time, and cortical surface area.[ref]
Note that since the publication of this Mendelian randomization study, the Journal of the American College of Cardiology came out with a long position paper on why this specific study shouldn’t be taken seriously in concluding that HMGCR inhibition could cause cognitive problems. However, be sure to read the conflict of interest section, where the authors of the article disclose consulting fees from a bunch of pharmaceutical companies.[ref]
Another Mendelian randomization study used genetic variants in HMGCR to determine the effects of statin-mimicking genetic variants. The study found that there was a causal relationship between lower HMGCR (statin-mimicking variants) and higher HbA1c and BMI.[ref] This study supports the link between statin use and increased risk of type 2 diabetes.
On the positive side, Mendelian randomization studies also point to the benefit of HMG-CoA reductase inhibition in preventing ovarian cancer and overall cancer risk. This risk reduction was not due to lower cholesterol levels, suggesting that the lower HMG-CoA levels affect cancer risk in other ways.[ref][ref]
The overall benefit of a small reduction in cancer risk may explain why statin use reduces all-cause mortality by 1% without having much effect on stroke or heart attack rates.[ref]
Genotype report: Statins and HMGCR
Related articles:
Reference List:
Akefe, Isaac O., et al. “Lipids and Secretory Vesicle Exocytosis.” Advances in Neurobiology, vol. 33, 2023, pp. 357–97. PubMed, https://doi.org/10.1007/978-3-031-34229-5_14.
Björkhem, Ingemar, and Steve Meaney. “Brain Cholesterol: Long Secret Life behind a Barrier.” Arteriosclerosis, Thrombosis, and Vascular Biology, vol. 24, no. 5, May 2004, pp. 806–15. PubMed, https://doi.org/10.1161/01.ATV.0000120374.59826.1b.
Byrne, Paula, et al. “Evaluating the Association Between Low-Density Lipoprotein Cholesterol Reduction and Relative and Absolute Effects of Statin Treatment: A Systematic Review and Meta-Analysis.” JAMA Internal Medicine, vol. 182, no. 5, May 2022, pp. 474–81. Silverchair, https://doi.org/10.1001/jamainternmed.2022.0134.
—. “Evaluating the Association Between Low-Density Lipoprotein Cholesterol Reduction and Relative and Absolute Effects of Statin Treatment: A Systematic Review and Meta-Analysis.” JAMA Internal Medicine, vol. 182, no. 5, May 2022, pp. 474–81. Silverchair, https://doi.org/10.1001/jamainternmed.2022.0134.
CDC. “Statins and Diabetes: What You Should Know.” Centers for Disease Control and Prevention, 30 Jan. 2023, https://www.cdc.gov/diabetes/library/features/Statins_Diabetes.html.
Climent, Elisenda, et al. “Hydrophilic or Lipophilic Statins?” Frontiers in Cardiovascular Medicine, vol. 8, 2021. Frontiers, https://www.frontiersin.org/articles/10.3389/fcvm.2021.687585.
Evans, Marcella A., and Beatrice A. Golomb. “Statin-Associated Adverse Cognitive Effects: Survey Results from 171 Patients.” Pharmacotherapy, vol. 29, no. 7, July 2009, pp. 800–11. PubMed, https://doi.org/10.1592/phco.29.7.800.
—. “Statin-Associated Adverse Cognitive Effects: Survey Results from 171 Patients.” Pharmacotherapy, vol. 29, no. 7, July 2009, pp. 800–11. PubMed, https://doi.org/10.1592/phco.29.7.800.
Familial Hypercholesterolemia | CDC. 16 June 2023, https://www.cdc.gov/genomics/disease/fh/FH.htm.
Fracassi, Anna, et al. “Statins and the Brain: More than Lipid Lowering Agents?” Current Neuropharmacology, vol. 17, no. 1, Jan. 2019, pp. 59–83. PubMed Central, https://doi.org/10.2174/1570159X15666170703101816.
—. “Statins and the Brain: More than Lipid Lowering Agents?” Current Neuropharmacology, vol. 17, no. 1, Jan. 2019, pp. 59–83. PubMed Central, https://doi.org/10.2174/1570159X15666170703101816.
Golomb, Beatrice A., et al. “Physician Response to Patient Reports of Adverse Drug Effects.” Drug Safety, vol. 30, no. 8, Aug. 2007, pp. 669–75. Springer Link, https://doi.org/10.2165/00002018-200730080-00003.
Jia, Zhenshan, et al. “Simvastatin Prodrug Micelles Target Fracture and Improve Healing.” Journal of Controlled Release : Official Journal of the Controlled Release Society, vol. 200, Feb. 2015, pp. 23–34. PubMed Central, https://doi.org/10.1016/j.jconrel.2014.12.028.
Kim, Jinkwon, et al. “Effect of Statins on Fasting Glucose in Non-Diabetic Individuals: Nationwide Population-Based Health Examination in Korea.” Cardiovascular Diabetology, vol. 17, no. 1, Dec. 2018, p. 155. BioMed Central, https://doi.org/10.1186/s12933-018-0799-4.
Ko, Min Jung, et al. “Time‐ and Dose‐Dependent Association of Statin Use With Risk of Clinically Relevant New‐Onset Diabetes Mellitus in Primary Prevention: A Nationwide Observational Cohort Study.” Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease, vol. 8, no. 8, Apr. 2019, p. e011320. PubMed Central, https://doi.org/10.1161/JAHA.118.011320.
Leduc, Valerie, et al. “Role of Rs3846662 and HMGCR Alternative Splicing in Statin Efficacy and Baseline Lipid Levels in Familial Hypercholesterolemia.” Pharmacogenetics and Genomics, vol. 26, no. 1, Jan. 2016, pp. 1–11. PubMed Central, https://doi.org/10.1097/FPC.0000000000000178.
Li, Rui, et al. “Effects of Plasma Lipids and Statins on Cognitive Function.” Chinese Medical Journal, vol. 131, no. 4, Feb. 2018, pp. 471–76. PubMed Central, https://doi.org/10.4103/0366-6999.225062.
Matyori, Amro, et al. “Statins Utilization Trends and Expenditures in the U.S. before and after the Implementation of the 2013 ACC/AHA Guidelines.” Saudi Pharmaceutical Journal : SPJ, vol. 31, no. 6, June 2023, pp. 795–800. PubMed Central, https://doi.org/10.1016/j.jsps.2023.04.002.
—. “Statins Utilization Trends and Expenditures in the U.S. before and after the Implementation of the 2013 ACC/AHA Guidelines.” Saudi Pharmaceutical Journal : SPJ, vol. 31, no. 6, June 2023, pp. 795–800. PubMed Central, https://doi.org/10.1016/j.jsps.2023.04.002.
—. “Statins Utilization Trends and Expenditures in the U.S. before and after the Implementation of the 2013 ACC/AHA Guidelines.” Saudi Pharmaceutical Journal : SPJ, vol. 31, no. 6, June 2023, pp. 795–800. PubMed Central, https://doi.org/10.1016/j.jsps.2023.04.002.
—. “Statins Utilization Trends and Expenditures in the U.S. before and after the Implementation of the 2013 ACC/AHA Guidelines.” Saudi Pharmaceutical Journal : SPJ, vol. 31, no. 6, June 2023, pp. 795–800. PubMed Central, https://doi.org/10.1016/j.jsps.2023.04.002.
Mollazadeh, Hamid, et al. “Effects of Statins on Mitochondrial Pathways.” Journal of Cachexia, Sarcopenia and Muscle, vol. 12, no. 2, Apr. 2021, pp. 237–51. DOI.org (Crossref), https://doi.org/10.1002/jcsm.12654.
—. “Effects of Statins on Mitochondrial Pathways.” Journal of Cachexia, Sarcopenia and Muscle, vol. 12, no. 2, Apr. 2021, pp. 237–51. DOI.org (Crossref), https://doi.org/10.1002/jcsm.12654.
Muldoon, M. F., et al. “Effects of Lovastatin on Cognitive Function and Psychological Well-Being.” The American Journal of Medicine, vol. 108, no. 7, May 2000, pp. 538–46. PubMed, https://doi.org/10.1016/s0002-9343(00)00353-3.
Na, Eonji, et al. “Time-Varying and Dose-Dependent Effect of Long-Term Statin Use on Risk of Type 2 Diabetes: A Retrospective Cohort Study.” Cardiovascular Diabetology, vol. 19, no. 1, May 2020, p. 67. PubMed, https://doi.org/10.1186/s12933-020-01037-0.
Peña, Jessica M., et al. “Statin Therapy and Risk of Fracture: Results from the JUPITER Randomized Clinical Trial.” JAMA Internal Medicine, vol. 175, no. 2, Feb. 2015, pp. 171–77. PubMed, https://doi.org/10.1001/jamainternmed.2014.6388.
Research, Center for Drug Evaluation and. “FDA Drug Safety Communication: Important Safety Label Changes to Cholesterol-Lowering Statin Drugs.” FDA, June 2019. www.fda.gov, https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-important-safety-label-changes-cholesterol-lowering-statin-drugs.
Rosoff, Daniel B., et al. “Mendelian Randomization Study of PCSK9 and HMG-CoA Reductase Inhibition and Cognitive Function.” Journal of the American College of Cardiology, vol. 80, no. 7, Aug. 2022, pp. 653–62. PubMed, https://doi.org/10.1016/j.jacc.2022.05.041.
Samaras, Katherine, et al. “Effects of Statins on Memory, Cognition, and Brain Volume in the Elderly.” Journal of the American College of Cardiology, vol. 74, no. 21, Nov. 2019, pp. 2554–68. ScienceDirect, https://doi.org/10.1016/j.jacc.2019.09.041.
—. “Effects of Statins on Memory, Cognition, and Brain Volume in the Elderly.” Journal of the American College of Cardiology, vol. 74, no. 21, Nov. 2019, pp. 2554–68. ScienceDirect, https://doi.org/10.1016/j.jacc.2019.09.041.
Schultz, Bob G., et al. “The Role of Statins in Both Cognitive Impairment and Protection against Dementia: A Tale of Two Mechanisms.” Translational Neurodegeneration, vol. 7, no. 1, Feb. 2018, p. 5. BioMed Central, https://doi.org/10.1186/s40035-018-0110-3.
—. “The Role of Statins in Both Cognitive Impairment and Protection against Dementia: A Tale of Two Mechanisms.” Translational Neurodegeneration, vol. 7, no. 1, Feb. 2018, p. 5. BioMed Central, https://doi.org/10.1186/s40035-018-0110-3.
Sidebottom, Abbey C., et al. “Trends in Prevalence of Guideline‐based Use of Lipid‐lowering Therapy in a Large Health System.” Clinical Cardiology, vol. 43, no. 6, Feb. 2020, pp. 560–67. PubMed Central, https://doi.org/10.1002/clc.23347.
Sooksawate, T., and M. A. Simmonds. “Effects of Membrane Cholesterol on the Sensitivity of the GABA(A) Receptor to GABA in Acutely Dissociated Rat Hippocampal Neurones.” Neuropharmacology, vol. 40, no. 2, 2001, pp. 178–84. PubMed, https://doi.org/10.1016/s0028-3908(00)00159-3.
“Statins Giving You Achy Muscles? Ask Your Doctor About These 4 Potential Fixes.” Cleveland Clinic, https://health.clevelandclinic.org/statins-giving-you-achy-muscles-ask-your-doctor-about-these-4-potential-fixes. Accessed 14 Dec. 2023.
Stroes, Erik S., et al. “Statin-Associated Muscle Symptoms: Impact on Statin Therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management.” European Heart Journal, vol. 36, no. 17, May 2015, pp. 1012–22. PubMed, https://doi.org/10.1093/eurheartj/ehv043.
Trapani, Laura, et al. “Regulation and Deregulation of Cholesterol Homeostasis: The Liver as a Metabolic ‘Power Station.’” World Journal of Hepatology, vol. 4, no. 6, June 2012, pp. 184–90. PubMed Central, https://doi.org/10.4254/wjh.v4.i6.184.
Yarmolinsky, James, et al. “Association Between Genetically Proxied Inhibition of HMG-CoA Reductase and Epithelial Ovarian Cancer.” JAMA, vol. 323, no. 7, Feb. 2020, pp. 646–55. PubMed Central, https://doi.org/10.1001/jama.2020.0150.
Young, A. Joyce, et al. “Coenzyme Q10: A Review of Its Promise as a Neuroprotectant.” CNS Spectrums, vol. 12, no. 1, Jan. 2007, pp. 62–68. PubMed, https://doi.org/10.1017/s1092852900020538.
Debbie Moon is the founder of Genetic Lifehacks. Fascinated by the connections between genes, diet, and health, her goal is to help you understand how to apply genetics to your diet and lifestyle decisions. Debbie has a BS in engineering from Colorado School of Mines and an MSc in biological sciences from Clemson University. Debbie combines an engineering mindset with a biological systems approach to help you understand how genetic differences impact your optimal health.