A recent research article in Science Immunology explains, at least in part, a question that has puzzled people throughout the pandemic: Why did SARS-CoV-2 result in severe disease for a very small percentage of young, healthy adults? The data was clear from the beginning of the pandemic that the elderly were at high risk, but elderly people have always been very susceptible to respiratory viruses. The question is why the virus is deadly for a small percentage of healthy, younger adults… One answer: TLR7 mutations.[ref]
Just a quick aside – this isn’t something that is new with SARS-CoV-2. We all have different genetic variants that impact our responses to different viruses. As a whole, a species survives new pathogens because of the huge diversity in immune responses. Thus, people can be more susceptible to certain pathogens but be resistant to others due to genetic variants. (Read more about viral susceptibility and genetics.)
This article digs into the TLR7 mutations that increase susceptibility to severe COVID-19. I want to be clear up front that this is likely not the only gene with mutations that causes an increased risk of mortality from SARS-CoV-2. Researchers are still working on the whole picture for genetic susceptibility, and other risk factors (age, metabolic health) also are very important.
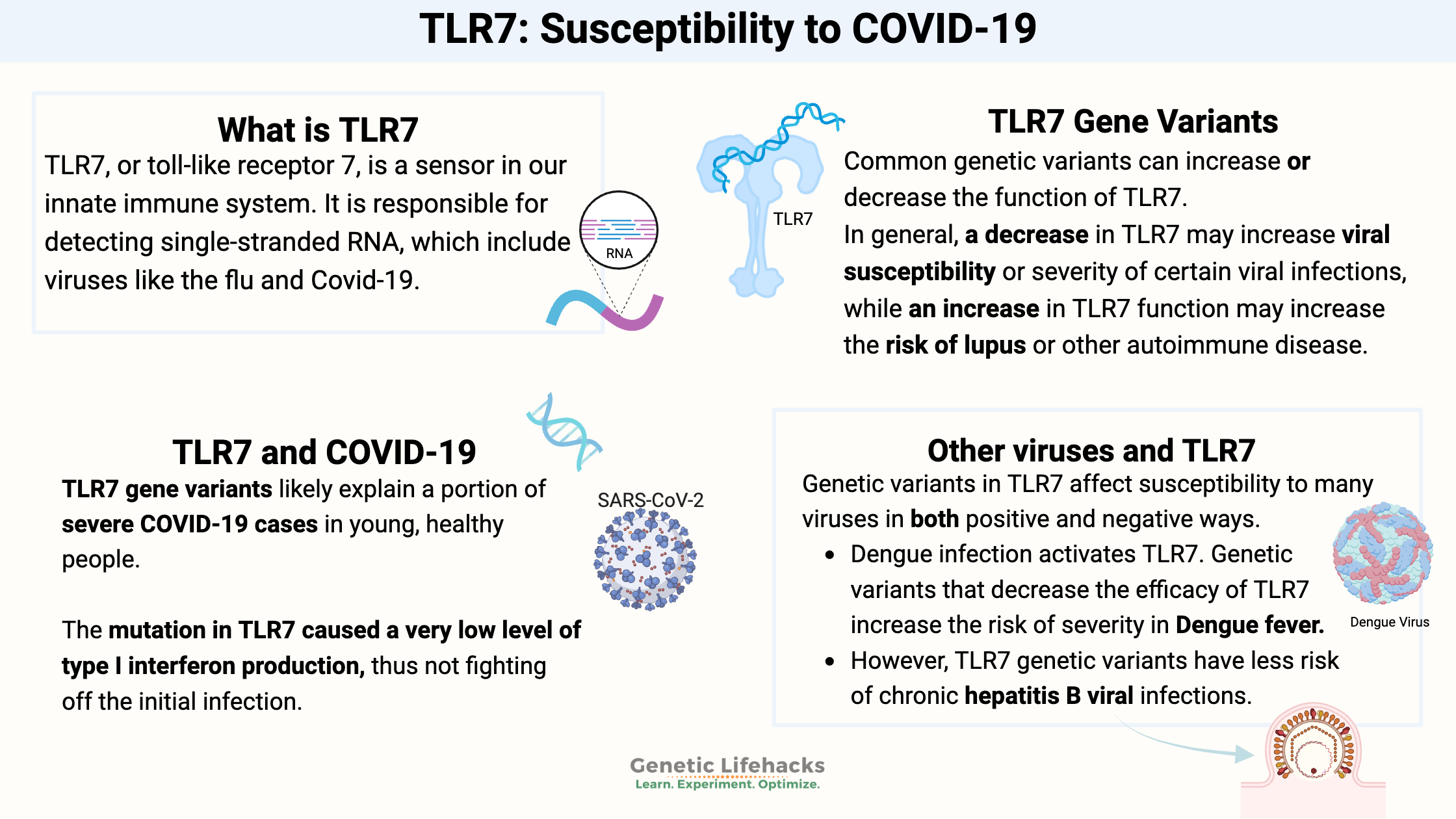
What is TLR7?
TLR7, or toll-like receptor 7, is a sensor in our innate immune system. It is responsible for detecting single-stranded RNA.
Hang on – what are single-stranded RNA viruses? Viruses can be encoded by RNA or DNA, single- or double-stranded.
Single-stranded RNA viruses include:[ref][ref]
- coronaviruses, including SARS-CoV-2
- Hantavirus
- Dengue virus
- Chikungunya
- hepatitis A
- West Nile virus
- influenza A
Your immune system cells, such as macrophages and dendritic cells, have a bunch of different toll-like receptors on them. They are named TLR1 through TLR13, and their role is to recognize different molecules found on pathogens. For example, some TLRs recognize lipopolysaccharides on Gram-negative bacteria and others recognized double- or single-stranded RNA viruses. Certain TLRs also recognized fungi, with others detecting parasitic protozoans.
Back to TLR7…
The TLR7 receptors are located primarily in B-cells and dendritic cells, which are types of immune cells that circulate. Additionally, low levels of TLR7 have been found in epithelial cells, such as the cells lining the lungs, and in liver cells.
When the TLR7 receptor is activated by a single-stranded RNA virus, it induces the body’s initial immune response to a virus. This response includes type I interferon as well as other inflammatory cytokines.[ref]
X-linked gene:
The TLR7 gene is located on the X-chromosome, so males only have one copy of the gene. Therefore, a mutation that inactivates one copy of the gene causes a complete knockout of TLR7 in men with the mutation.
Women, on the other hand, have two X chromosomes. Normally, one of the X-chromosomes is silenced in cells, so some cells may offer different TLR7 responses than others for women who are heterozygous for TLR7 mutations. But taken together, TLR7 mutations in women offer an intermediate phenotype.
TLR7 and COVID-19:
The recent Science Immunology report that I mentioned above explained that researchers have discovered that TLR7 mutations likely explain a portion of severe COVID-19 cases in young, healthy people.[ref]
The mutation in TLR7 caused a very low level of type I interferon production, thus not fighting off the initial infection. The researchers also showed that a subset of B cell lines from severe (young, healthy males) COVID-19 patients didn’t respond to TLR7 stimulation.
The study concludes “Overall, X-linked recessive TLR7 deficiency is a highly penetrant genetic etiology of critical COVID-19 pneumonia, in about 1.8% of male patients below the age of 60 years.”[ref]
In a similar study published in the Journal of the American Medical Association in August 2020, researchers reported on two pairs of brothers, ages 21-32, who had severe Covid that led to ICU or death. The genetic testing on these four men young men showed that they all carried rare mutations in the TLR7 gene.[ref]
The human immune system (and all animals, really) has many different methods of detecting pathogens of different types. Thus, TLR7 is not the only way that the immune system likely recognizes SARS-CoV-2 as a pathogen. TLR3 may also be activated, as well as RIG-I-like receptors.[ref]
Genetics studies on severe COVID (in healthy adults) showed that mutations in TLR3 and a type I interferon gene can also cause severe COVID-19. [ref]
Are mutations in interferon-related genes the cause of COVID deaths in young, healthy adults?
The frequency of the TLR7, TLR3, and other interferon gene mutations is low – around 1 in 1,000 for the TLR3 mutation. The TLR7 mutations are even rarer. Keep in mind, though, that there are multiple loss-of-function mutations in these genes. So you may have 4 or 5 people out of a thousand with rare loss of function mutations. But, the frequency of young, healthy adults succumbing to COVID-19 is also very low.
Other viruses and TLR7:
TLR7 is important in detecting other RNA viruses as well. Genetic variants in TLR7 affect susceptibility to these viruses in both positive and negative ways.
- Dengue infection activates TLR7. Genetic variants that decrease the efficacy of TLR7 increase the risk of severity in Dengue fever.[ref]
- Women with certain TLR7 genetic variants have less risk of chronic hepatitis B viral infections.[ref]
- People carrying certain variants in TLR7 are much more likely to spontaneously clear hepatitis C and not progress to liver disease.[ref]
TLR7 and Lupus:
While TLR7 is supposed to detect and respond to RNA viruses, sometimes things go awry and overactivation of the immune response causes problems.
Lupus is a complex autoimmune disease that can affect multiple systems in the body. In lupus, the body creates autoantibodies against certain self-nucleic acids (antinuclear antibodies). These autoantibodies cause local inflammation and initiate an innate immune response, part of which is due to TLR7 activation.
Thus, TLR7 and its stimulation of interferon, is at the heart of the overactive immune response, at least for some people, with lupus.[ref][ref][ref]
Interestingly, the fact that TLR7 is located on the X-chromosome may play a key role in autoimmune diseases such as lupus. Normally in women, one copy of the X-chromosome is silenced in cells. (Not always the same copy is silenced in all cells – so there can be tissue-specific changes with X-chromosome silencing.)
Related article: Genetics of Lupus: Understanding how your genes increase lupus risk
Women are at a much higher risk of lupus than men are, and people with Kleinfelter syndrome (XXY) are also at a high risk of lupus. Researchers have found that women with lupus may not have the second X-chromosome silenced in B-cells, leading to greater TLR7 expression.[ref]
Vaccines and TLR7:
Activating TLR7 increases immune response — necessary when fighting off a virus and also needed in creating an immune system response to a vaccine.
Interestingly, a synthetic phospholipid has been developed to add to flu vaccines. It increases the immune response by binding to and activating TLR7.[ref][ref]
Other clinical trials using mRNA vaccines include “a palmitic acid-modified TLR7/8 agonist R848 (C16-R848), coated with a lipid-polyethylene glycol (lipid-PEG) shell”.[ref][ref]
TLR7 Genotype Report
Lifehacks:
Obviously, most people won’t have a rare TLR7 mutation. But you can do your best to prevent illnesses, such as COVID-19, by being in good health.
I have a whole series of articles on the research studies on treatments and the prevention of comorbidities for SARS-CoV-2:
Research Roundup: Covid-19 prevention and mitigation
Related Articles and Genes:
Genetic susceptibility to viruses
Your genetic variants shape your immune system and give you superpowers against some pathogens – and perhaps more susceptible to others.
Vitamin D, Genes, and Your Immune System
Vitamin D is more than just a ‘vitamin’. It is actually a hormone that is essential to so many processes in your body – including your immune system.
Acute Respiratory Distress Syndrome Genes
This is article explains what happens to the body in ARDS, and it goes into the genetic variants that increase or decrease the risk of ARDS (due to all causes – not just COVID-19). ARDS is a ‘syndrome’, and thus a collection of symptoms rather than a disease.
COVID-19 & Genetics: Who gets sick and why?
Not everyone gets sick when exposed to the SARS-CoV-2 virus. While there are many factors that come into play here, research points to genetics as playing a role. Several good genetic studies have recently been released showing which genes are important in COVID-19 susceptibility. Learn more and check your genes.
References:
Asano, Takaki, et al. “X-Linked Recessive TLR7 Deficiency in ~1% of Men under 60 Years Old with Life-Threatening COVID-19.” Science Immunology, vol. 0, no. 0, p. eabl4348. science.org (Atypon), https://doi.org/10.1126/sciimmunol.abl4348.
—. “X-Linked Recessive TLR7 Deficiency in ~1% of Men under 60 Years Old with Life-Threatening COVID-19.” Science Immunology, vol. 0, no. 0, p. eabl4348. science.org (Atypon), https://doi.org/10.1126/sciimmunol.abl4348.
Buschow, Sonja I., et al. “TLR7 Polymorphism, Sex and Chronic HBV Infection Influence Plasmacytoid DC Maturation by TLR7 Ligands.” Antiviral Research, vol. 157, Sept. 2018, pp. 27–37. ScienceDirect, https://doi.org/10.1016/j.antiviral.2018.06.015.
Celhar, Teja, and Anna-Marie Fairhurst. “Modelling Clinical Systemic Lupus Erythematosus: Similarities, Differences and Success Stories.” Rheumatology (Oxford, England), vol. 56, no. suppl_1, Apr. 2017, pp. i88–99. PubMed, https://doi.org/10.1093/rheumatology/kew400.
COVID-19 Provisional Counts – Weekly Updates by Select Demographic and Geographic Characteristics. 8 Sept. 2021, https://www.cdc.gov/nchs/nvss/vsrr/covid_weekly/index.htm.
dos Santos, B. P., et al. “TLR7/8/9 Polymorphisms and Their Associations in Systemic Lupus Erythematosus Patients from Southern Brazil.” Lupus, vol. 21, no. 3, Mar. 2012, pp. 302–09. PubMed, https://doi.org/10.1177/0961203311425522.
Dutta, Sudip Kumar, and Anusri Tripathi. “Association of Toll-like Receptor Polymorphisms with Susceptibility to Chikungunya Virus Infection.” Virology, vol. 511, Nov. 2017, pp. 207–13. PubMed, https://doi.org/10.1016/j.virol.2017.08.009.
Fakhir, Fatima-Zohra, et al. “Genetic Variations in Toll-like Receptors 7 and 8 Modulate Natural Hepatitis C Outcomes and Liver Disease Progression.” Liver International: Official Journal of the International Association for the Study of the Liver, vol. 38, no. 3, Mar. 2018, pp. 432–42. PubMed, https://doi.org/10.1111/liv.13533.
Goff, Peter H., et al. “Synthetic Toll-Like Receptor 4 (TLR4) and TLR7 Ligands as Influenza Virus Vaccine Adjuvants Induce Rapid, Sustained, and Broadly Protective Responses.” Journal of Virology, vol. 89, no. 6, Jan. 2015, pp. 3221–35. PubMed Central, https://doi.org/10.1128/JVI.03337-14.
Hanna Kazazian, Noël, et al. “Lupus Autoimmunity and Metabolic Parameters Are Exacerbated Upon High Fat Diet-Induced Obesity Due to TLR7 Signaling.” Frontiers in Immunology, vol. 10, Sept. 2019, p. 2015. PubMed Central, https://doi.org/10.3389/fimmu.2019.02015.
Hise, Amy G., et al. “Association of Symptoms and Severity of Rift Valley Fever with Genetic Polymorphisms in Human Innate Immune Pathways.” PLoS Neglected Tropical Diseases, vol. 9, no. 3, Mar. 2015, p. e0003584. PubMed Central, https://doi.org/10.1371/journal.pntd.0003584.
Islam, Mohammad Ariful, et al. “Adjuvant-Pulsed MRNA Vaccine Nanoparticle for Immunoprophylactic and Therapeutic Tumor Suppression in Mice.” Biomaterials, vol. 266, Jan. 2021, p. 120431. PubMed, https://doi.org/10.1016/j.biomaterials.2020.120431.
Kawasaki, Aya, et al. “TLR7 Single-Nucleotide Polymorphisms in the 3’ Untranslated Region and Intron 2 Independently Contribute to Systemic Lupus Erythematosus in Japanese Women: A Case-Control Association Study.” Arthritis Research & Therapy, vol. 13, no. 2, 2011, p. R41. PubMed Central, https://doi.org/10.1186/ar3277.
Kim, You-Me, and Eui-Cheol Shin. “Type I and III Interferon Responses in SARS-CoV-2 Infection.” Experimental & Molecular Medicine, vol. 53, no. 5, May 2021, pp. 750–60. www.nature.com, https://doi.org/10.1038/s12276-021-00592-0.
Long, Yiru, et al. CoVac501, a Self-Adjuvanting Peptide Vaccine Conjugated with TLR7 Agonists, against SARS-CoV-2 Induces Protective Immunity. 11 Apr. 2021, p. 2021.04.10.439275. bioRxiv, https://www.biorxiv.org/content/10.1101/2021.04.10.439275v1.
Lvov, Dimitry Konstantinovich, et al. “Single-Stranded RNA Viruses.” Zoonotic Viruses in Northern Eurasia, 2015, pp. 135–392. PubMed Central, https://doi.org/10.1016/B978-0-12-801742-5.00008-8.
Mackelprang, Romel D., et al. “Host Genetic and Viral Determinants of HIV-1 RNA Set Point among HIV-1 Seroconverters from Sub-Saharan Africa.” Journal of Virology, vol. 89, no. 4, Dec. 2014, pp. 2104–11. PubMed Central, https://doi.org/10.1128/JVI.01573-14.
Modrow, Susanne, et al. “Viruses with Single-Stranded, Positive-Sense RNA Genomes.” Molecular Virology, edited by Susanne Modrow et al., Springer, 2013, pp. 185–349. Springer Link, https://doi.org/10.1007/978-3-642-20718-1_14.
Mukherjee, Saikat, and Anusri Tripathi. “Contribution of Toll like Receptor Polymorphisms to Dengue Susceptibility and Clinical Outcome among Eastern Indian Patients.” Immunobiology, vol. 224, no. 6, Nov. 2019, pp. 774–85. PubMed, https://doi.org/10.1016/j.imbio.2019.08.009.
—. “Contribution of Toll like Receptor Polymorphisms to Dengue Susceptibility and Clinical Outcome among Eastern Indian Patients.” Immunobiology, vol. 224, no. 6, Nov. 2019, pp. 774–85. PubMed, https://doi.org/10.1016/j.imbio.2019.08.009.
Petes, Carlene, et al. “The Toll for Trafficking: Toll-Like Receptor 7 Delivery to the Endosome.” Frontiers in Immunology, vol. 8, 2017, p. 1075. Frontiers, https://doi.org/10.3389/fimmu.2017.01075.
Pisetsky, David S. “The Central Role of Nucleic Acids in the Pathogenesis of Systemic Lupus Erythematosus.” F1000Research, vol. 8, Apr. 2019, p. F1000 Faculty Rev-368. PubMed Central, https://doi.org/10.12688/f1000research.17959.1.
Sato-Kaneko, Fumi, et al. “A Novel Synthetic Dual Agonistic Liposomal TLR4/7 Adjuvant Promotes Broad Immune Responses in an Influenza Vaccine With Minimal Reactogenicity.” Frontiers in Immunology, vol. 11, 2020, p. 1207. Frontiers, https://doi.org/10.3389/fimmu.2020.01207.
van der Made, Caspar I., et al. “Presence of Genetic Variants Among Young Men With Severe COVID-19.” JAMA, vol. 324, no. 7, Aug. 2020, pp. 663–73. PubMed, https://doi.org/10.1001/jama.2020.13719.
Zhang, Qian, et al. “Inborn Errors of Type I IFN Immunity in Patients with Life-Threatening COVID-19.” Science, vol. 370, no. 6515, Oct. 2020, p. eabd4570. science.org (Atypon), https://doi.org/10.1126/science.abd4570.